 | ||
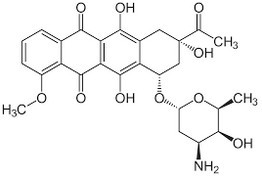
| Daunorubicin, , is a DNA-targeting anticancer drug that is used as chemotherapy, mainly for patients with leukemia. It has also been shown to have anticancer effects in monotherapy or combination therapy in solid tumors and advanced HIV associated Kaposi's sarcoma |
| Daunorubicin
is an anthracycline antibiotic and antineoplastic agent. It acts by
inhibiting cellular reproduction through interference with DNA
replication although it may contribute to the induction of cell death
by increasing oxidative stress through the generation of reactive
oxygen species and free radicals. As an antineoplastic agent,
daunorubicin carries significant toxicities including cytopenias,
hepatotoxicity, and extravasation reactions. Like other anthracyclines, daunorubicin also exhibits cardiotoxicity in proportion with the cumulative dose received over time. | Myocardial
toxicity manifested in its most severe form by potentially fatal
congestive heart failure may occur either during therapy or months to
years after termination of therapy. The incidence of myocardial
toxicity increases after a total cumulative dose exceeding 400 to 550
mg/sq m in adults, 300 mg/sq m in children more than 2 years of age, or
10 mg/kg in children less than 2 years of age. more info https://pubchem.ncbi.nlm.nih.gov/compound/Daunorubicin |
Production of daunorubicine Daunorubicin is produced through fermentation of specific Streptomyces bacteria species, generally Streptomyces peucetius or other mutant obtained by selection or gene modification so as to increase the yield of daunorubicin or optimize production conditions.The first trials were done in 1962 by researcher of an italian company Farmitalia SPA who took the first patent . 1. Fermentation Medium Preparation: A nutrient-rich fermentation medium is prepared, containing sources of carbon (e.g., glucose, sucrose,starch..), nitrogen (e.g., soybean meal, ammonium salts...), and essential minerals. Cultivation: The bacterial culture is grown in the medium under controlled conditions (temperature, pH, aeration). The growth phase is critical for maximizing the biosynthesis of daunorubicin. Induction of Secondary Metabolism: Once the bacteria reach a specific growth stage, secondary metabolism is triggered to produce daunorubicin as part of the organism's natural defense mechanism. The whole fermentation process last for 7 days or more .More details on fermentation process in US patent 4,012,284 (Farmitalia SPA continuation of patent US32284763 filed November 12, 1963 ) (see also US04/404,550 ) .Other conditions in 1983 US4592999 .Since this patent things have changed .For example in (China Patent CN-112646852 filed 2021 ) where the bacteria used for fermentation are streptomyces baumannii, streptomyces griseus ,streptomyces coeruleorubidus . | 2 Extraction of daunorubicin After fermentation, daunorubicin is extracted from the fermentation broth The cells are lysed to release intracellular daunorubicin by a suitable chemical treatment with addition of a tailored organic solvent .After that daunorubicin is no longer within the cells but in solution.The daunorubicine concentration is quite low (generally less than 5 gr/liter of broth) . After mycellium removal by filtration daunrubicine is adsorbed on a support so as to remove majority of by products which are eluted .The daunorubine adsorbed is selectively desorbed by a mixture of solvent . The obtained solution is purified, concentrated and crystallized so as to obtaine daunorubicine in its base form . Purification is done on daunorubin hydrochloride by selective crystallisation using mixture of organic solvents . The complexity and the low productivity of the whole process explains why the price of daunorubicin is not cheap . |
DAUNORUBICINE USAGE Several companies worldwide produce daunorubicin for the medical market ,. Major manufacturers include: Sanofi under the brand name Cerubidine, Pfizer (Daunoblastin)- Sun Pharmaceutical Industries Teva Pharmaceuticals , a generic drug manufacturer (brand name daunorubicin) Cipla and Zydus Cadila (Norubin) - Indian pharmaceutical companies active in producing generic Major part of production is used in the medical market to treat mainly some forms of leukemia .For this usage daunorubine is sold as a mixture with a sugar (20 mg of Daunorubicine + 200 mg of mannitol) ready for intravenous injection after dilution in 5 to 10 ml of sterile water at 0.9% of NaCl . Daunorubicine is also sold as a mixture with other product to improve treatment efficiency by a better release control and conjugated action with another anti mitotic drug .For example (as said in US8022279 filed 2005) a composition for parenteral administration to a subject which composition comprises liposomes having associated therewith daunorubicin and cytarabine (cytosine arabinoside) in a mole ratio of daunorubicin:cytarabine of about 1:5 so that said mole ratio is maintained in the blood for at least one hour after administration to the subject. Vyxeos (liposomal daunorubicin and cytarabine) is marketed by Jazz Pharmaceuticals, an Ireland-based biopharmaceutical company. Daunorubicine can be alos used in conjuction with other antimitotic drug .For example to treat special form of leukemia daunorubicine is associated with cytarabine and venetoclax (see on going 2024 China phase 2 clinical trial ) Daunorubicine ( as citrate salt ) is also marketed as a mixture with liposone under the trade name DaunoXomeŽ . The identification of liposome formulations capable of delivering their contents to solid tumors in vivo is contingent upon the development of an active loading technique for entrapping a gamma emitter Using labeled liposomes, various formulations are screened for their ability to deliver entrapped materials to solid tumors selectively. With this labeling approach, a wide range of compositions and physical characteristics (size, phase transition temperature, surface charge, etc.) can evaluated for their abilities to remain intact in the circulation for prolonged periods as well as to deliver and release their entrapped contents selectively within solid tumors in vivo. An example of formulation consist of distearolyphosphatidylcholine (DSPC) and cholesterol in a 2:1 mole ratio with diameters less than 100 nm. It is specifically designed to improve the delivery of daunorubicin to tumor cells while reducing its impact on healthy tissues. DaunoXome is approved for the treatment of advanced HIV-associated Kaposi's sarcoma It is also possible to buy Daunorubicin for scientific research , mainly to modify the molecule to get other anti tumor antibiotics ,for example Detorubicine CAS 66211-92-55 , or 4-Demethoxydaunorubicin (alias Idarubicin see it's chemical synthesis ) ) Daunorubicine can be ordered directly from internet through various broker in pure form without any adjuvant (see a typical analysis ) As indicated before the price of daunorubicine is quite high (order of magnitude 90 euros / 25 mg) |     |
| The
chemical synthesis of daunorubicin, is a challenging process due to its
intricate molecular structure, which includes a tetracyclic quinone
nucleus attached to a sugar moiety (daunosamine). Total chemical synthesis is also possible but typically used for research purposes rather than commercial production due to its complexity. This complexity is exacerbed by the stereochemical control required for the sugar moiety and anthracycline core . Chemical synthesis of daunorubicine and analogs could follow the following scheme , each step being well documented in chemical literature : ** Construction of the Anthracycline Core: The core of daunorubicin is based on a tetracyclic anthraquinone skeleton. This step typically involves: * Diels-Alder Reaction: Starting with simple aromatic precursors, a cycloaddition is used to construct the fused ring system. *Functionalization: Subsequent oxidation and hydroxylation reactions introduce the hydroxy groups characteristic of the quinone and phenolic rings. ** Introduction of the Daunosamine Sugar Moiety: Daunosamine, a 3-amino-2,3,6-trideoxy sugar, is synthesized separately. Key steps include: Glycosylation reactions to form the sugar framework. Reductive amination to introduce the amino group. ** Glycosylation of the Anthracycline Core: The daunosamine sugar is attached to the anthracycline nucleus through a glycosidic bond at the C-7 position. This is achieved via: Activation of the sugar (e.g., using a trichloroacetimidate or a similar leaving group). Coupling with the anthraquinone scaffold under acidic or catalytic conditions. The total synthesis needs a lot of steps using expansive intermediates and it is not obvious that total cost per gram of final product will be cheaper than using fermentation route .So far no company has take any patent to protect this way of getting daunoramicin or anlaogs |  |
| It
is easier to chemically modify daunorubicine to get other
products ,for example to make 4-Demethoxydaunorubicin (Idarubicine) from daunorubicin 4-Demethoxydaunorubicin (idarubicin) is an analog of daunorubicin The absence of a methoxy group at position 4 of the anthracycline structure gives the compound a high lipophilicity, which results in an increased rate of cellular uptake compared with other anthracyclines. In combination with cytosine arabinoside 4-demethoxydaunorubicine is the current first line therapy of acute myeloid Available methods for the chemical synthesis of 4-demethoxydaunorubicin (idarubicin) are generally based on the coupling of the aglycone of the compound (i.e. the non-sugar component) and the protected and activated daunosamine (i.e. 3-amino-2,3,6-trideoxy-L-lyxo-hexose; the sugar component) in the presence of silver triflate (AgOSO2CF3), trimethylsilyl-triflate ((CH3)3SiOSO2CF3), or a mercuric oxide-mercuric bromide system (HgO—HgBr2). The aglycone may, for example, be synthesized using either anthracenetetrone or isobenzofurane as starting material. However, such synthesis methods are complex due to the creation of optically active centers at carbons C7 and C9. A simpler synthesis is shown (US patent 8846882) .It requires 5 chemicals steps to get Idarubicine from daunorubicin . | 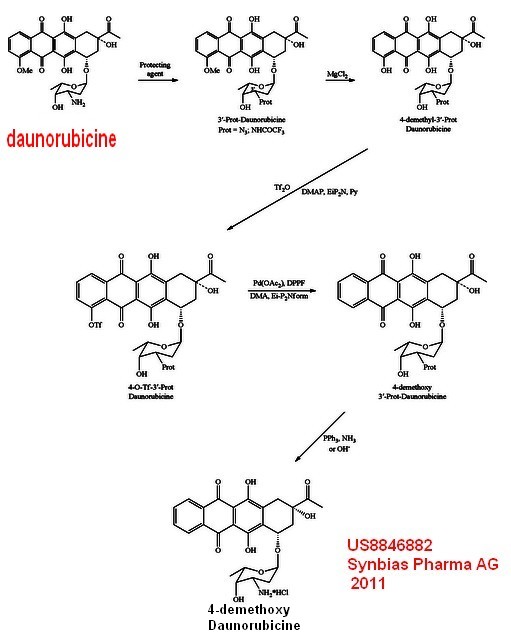 |