Preparation of formaldehyde
Abstract
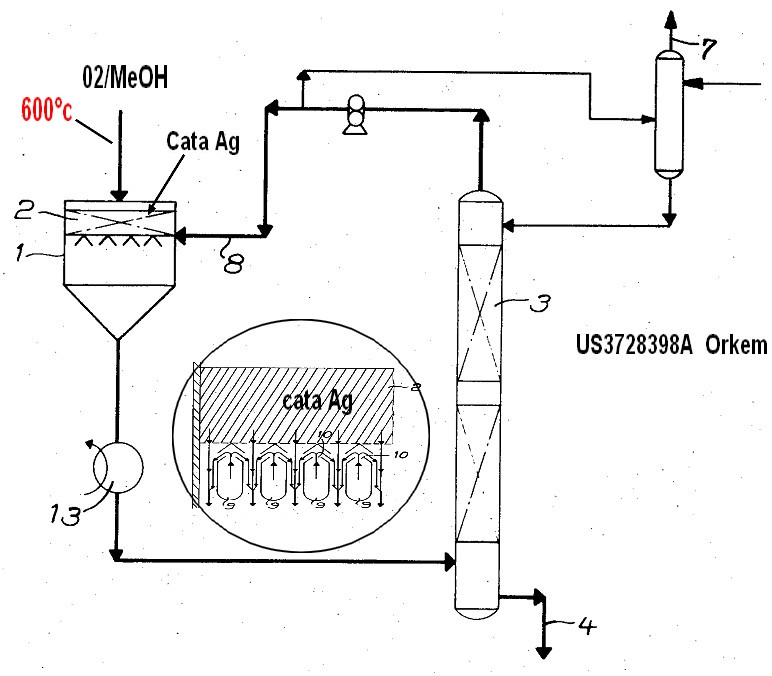
This invention relates to an improved process for the preparation of formaldehyde by oxidising methanol by air in the presence of a silver catalyst and is characterised in that the gases leaving the installation after separation of the condensable products, viz. water, methanol and formaldehyde, are at least partly reinjected in the cold state into the gaseous mixture leaving the catalyst layer. The present invention relates to the preparation of formaldehyde by the known process of oxidising methanol by air in the presence of a silver catalyst. In this known preparation, a gaseous mixture of methanol and air is passed at a suitable temperature over a silver catalyst. The reaction products leave the catalyst layer at a temperature between approximately 500*C and 650*C. These gases also contain the methanol which has not reacted, as well as the desired formaldehyde, water, nitorgen, carbon monoxide, carbon dioxide and hydrogen. It was proved nitrogen, long time ago that in such a gaseous mixture, the temperature of which is between approximately 500*C and 600*C, a certain number of chemical reactions are liable to develop which are considered as parasitic with respect to the main formation reaction of the formaldehyde and which it is generally desired to minimise or suppress. To this end, various methods have been advocated which can be summed up more particularly in the use of an indirect heat exchanger and in the use of atomization of water or a diluted solution of formol. Naturally, it was the aim of the various known methods to lower the temperature of the gases leaving the catalyst layer as quickly as possible to a value lower than 350*C - 450*C. These methods of treating hot gases have various drawbacks and it is an object of the invention to avoid or minimise these drawbacks as far as possible. According to the present invention, a method of preparing formaldehyde by oxidising methanol by air in the presence of a silver catalyst is characterised in that the gases leaving the plant after separation from the condensable products (water, methanol and formaldehyde) ARE AT LEAST partly reinjected in the cold state into the gaseous mixture leaving the catalyst layer. The reinjected gases contain, more particularly, nitrogen, carbon dioxide, carbon monoxide and hydrogen. These gases are referred to as ''''tail'''' gases, and are cold, since they are obtained after treating the gaseous mixture leaving the catalyst layer by known methods having as an object, the condensation or absorption of the condensable chemical products contained in this gaseous mixture. They are, on the other hand, only of small value and are usually rejected to atmosphere. However, used according to the invention, they allow the yield from a plant for preparing the formaldehyde to be substantially improved. This improvement in the yield is carried out following a complex mechanism which has not yet been entirely elucidated. It is caused certainly partly by the rapid cooling of the gaseous mixture leaving the catalyst layer, in which the synthesis of the formal dehyde is carried out: for this reason, it will be apparent that the reinjection of the tail gases must be effected as near as possible to the last layer of the catalyst bed and that it must be ensured, if maximum improvement is to be obtained, that a good distribution of the gases injected into the gaseous mixture leaving the catalyst is effected. But it is also certain that the improvement of yield is caused partly by the composition of the said tail gas. In order to obtain maximum improvement of yield, it has been found, on the other hand, that it was necessary to inject a quantity of tail gas into the gaseous mixture leaving the catalyst layer, representing per molecule of formol (formaldehyde) prepared approximately 1.7 times the quantity of tail gases produced. This is why, according to the invention, such a quantity of tail gas will be made to circulate and be reinjected into the gaseous mixture and that the gaseous circuit will be cleared from the excess gases produced as the reaction takes place. Amongst the other advantages of the method according to the invention may be mentioned: 1. The increase in the volume of inert gas resulting from the recirculation enables the evaporation of a large part of the water and methanol situated at the base of the first absorption tower, which is a particularly interesting advantage when ureaformaldehyde concentrates are to be produced by absorption by means of solutions of urea. 2. This same increase of the gaseous volume leaving the reactor facilitates thermal recuperation of the reaction heat by exchange, whilst avoiding premature condensation in the exchangers, on account of the partial decrease in pressure of the condensable part. 3. The invention can be adapted immediately to methods using a circuit operating at a pressure lower than atmospheric pressure throughout the line with synthesis of transport for the gases situated at the tail of the installation. Only the calibre of the compressor or ventilator used is to be increased.
Patent Citations (1)
Cited By (4)
Similar Documents
Priority And Related Applications
***************************************************************
Priority 1984-04-12 • Filed 1984-04-12 • Published 1994-08-17
Claims (1)
In the method for producing a 0% by weight formaldehyde solution, the amount of air per mol of the raw material methanol is 1.5 to
The total amount of water was 2.0 mol and the amount of water corresponding to the amount of water vapor added to the raw material gas and the amount of water necessary to dilute the product formaldehyde solution was added as absorption water from the top of the absorption tower,
In addition, the methanol concentration is 0.1 to 3.0 from the upper stage of the absorption tower.
Wt%, formaldehyde concentration of 0.1-1.0 wt% aqueous solution of the composition of 0.2 ~ per mol of the starting methanol
A method for producing an aqueous formaldehyde solution, which comprises extracting as a 0.8 mol side stream, adding this to a raw material gas, and reacting at a temperature of 580 to 680 ° C.
Description
translated from Japanese
DETAILED DESCRIPTION OF THE INVENTION 1) Field of Industrial Application The present invention relates to a method for producing a formaldehyde aqueous solution having a low methanol content by catalytically reacting a raw material gas containing methanol, air and water on a silver catalyst.
2) Conventional technology As a method for producing formaldehyde by oxidative dehydrogenation of methanol, a raw material methanol and air were further obtained by reacting a raw material gas obtained by adding steam and waste gas if necessary on a silver catalyst. It is common to recover the reaction heat from the reaction gas with a boiler, if necessary, and then introduce it into a water absorption tower to recover formaldehyde as an aqueous solution. In this case, since methanol is used in excess of air, unreacted residual methanol is generally mixed in the obtained formaldehyde. The residual methanol itself contributes to the stabilizing effect of the formaldehyde solution, but the excessive presence of methanol is wasteful, and in recent years, it has been required to produce an aqueous formaldehyde solution having a low methanol content.
Therefore, usually, a method is employed in which the amount of residual methanol in the reaction product gas is reduced by increasing the air / methanol molar ratio to about 1.8 to obtain formaldehyde having a low methanol content. In this case, avoid the explosion range and suppress the reaction temperature. Therefore, although steam is usually added to the raw material gas, this steam remains in the reaction product gas and is condensed in the absorption tower to reduce the concentration of the product formaldehyde aqueous solution. Therefore, the concentration of the formaldehyde aqueous solution produced by this method is limited to about 45% by weight.
On the other hand, as an effective means for reducing the methanol content of the product formaldehyde aqueous solution and increasing the formaldehyde concentration, there is a so-called waste gas circulation method in which a part of the absorption tower waste gas is added to the raw material gas. Is also a practically feasible product The concentration of formaldehyde aqueous solution is 55
Up to about wt%.
Further, when the air / methanol ratio of the raw material gas is increased, the combustion reaction of methanol and other side reactions tend to proceed and the formaldehyde selectivity tends to be lowered. Therefore, it is preferable to make the air / methanol ratio as low as possible.
3) Problems to be Solved by the Invention In order to solve the above-mentioned drawbacks, the present invention has an air / methanol molar ratio of the raw material gas of 1.5 to 1 when adding only steam to the raw material gas. About 6 or about 1.6 to 1.8 when waste gas is added to the raw material gas, and a formaldehyde aqueous solution having a low methanol content and a high formaldehyde concentration while reacting under milder conditions than conventional methods. To provide a method of manufacturing.
4) Means for solving the problem That is, in the present invention, a raw material gas containing methanol, air and steam is subjected to a catalytic reaction on a silver catalyst, and the obtained reaction product gas is contacted with water to remove formaldehyde in the product gas. Absorb in water, formaldehyde concentration 37 ~ 6 from the bottom of the absorption tower
In a method for producing a 0% by weight formaldehyde solution, water in an amount corresponding to water vapor added to a raw material gas,
Add the total amount of water necessary for diluting the product formaldehyde solution from the top of the absorption tower as absorbed water, and from the upper stage of the absorption tower, methanol concentration 0.1-3.0% by weight, formaldehyde concentration 0.1-. This is a method of producing formaldehyde by extracting an aqueous solution having a composition of 1.0% by weight as a side stream in an amount corresponding to water vapor added to a raw material gas, adding this to the raw material gas and reacting them.
5) Operation The raw material gas in the present invention contains methanol, air and water vapor, and if necessary, an inert gas such as a waste gas after the recovery of formaldehyde can be added to the reaction.
The composition of the source gas is methanol 1 if no waste gas is added.
Empty 1.5-2.0 mol per mol, preferably 1.6-
It is 1.8 moles, steam is 0.2 to 0.8 moles, and preferably 0.4 to 0.6 moles. When a waste gas is added, the empty space is 1.6 to 2.2 mol, preferably 1.8 to 2.0 mol, and water vapor is 0.2 to 0.6 mol, preferably 0.2 to 0. 5 mol, waste gas 0.2 to 2.0 mol, preferably 0.4 to 1.5 mol.
A raw material gas having such a composition is introduced onto an ordinary catalyst for producing formaldehyde such as electrolytic silver, and the temperature is 580 to 680 ° C., preferably 620 to 650 ° C., and the linear velocity is 0.8 to 2.0 m / se.
c, preferably 1.0 to 1.5 m / sec.
The reaction product gas obtained was formaldehyde 15-25.
%, Temperature 580-6 containing 0.2-2.0% methanol
It is a high temperature gas of 80 ° C., and therefore, it is usually passed through a multi-tube boiler installed directly below the reactor to obtain a temperature of 120-
After cooling to 160 ° C, it is introduced into an absorption tower.
The absorption tower is divided into three parts, the upper part, the middle part and the lower part, and the upper part is the plate tower, the middle part and the lower part are the packed towers. Water is supplied from the top of the tower and formaldehyde in the reaction product gas introduced from the bottom of the tower is absorbed. In the packed towers in the middle and lower parts of the tower, the absorbed water is withdrawn from the bottom of the tower to the outside as a side stream, cooled, and then circulated again to the top of each tower. Due to such external cooling, the temperature of the lower packed tower is 60 to 80 ° C, and the temperature of the middle packed tower is 20 to 30 ° C.
Kept at ℃. As a result, most of the formaldehyde and part of the methanol are absorbed and condensed in the lower packed column, and almost all of the formaldehyde and half of the tanol are absorbed in the middle packed column.
From the top of the upper tray tower, which is the top layer of the absorption tower, the amount of water necessary for diluting the aqueous formaldehyde solution that is withdrawn from the bottom of the tower as a product is usually introduced as absorbed water. 600-2000 ppm formaldehyde in the overhead gas released, 1
500-5000ppm methanol and methyl formate 7
A loss of 0.00 to 3000 ppm is included. In the present invention, in addition to the amount of water necessary for diluting the product formaldehyde aqueous solution from the top of the plate tower, water is added in an amount corresponding to the water added to the raw material gas, and the upper plate column (total plate number 10 to 10). 25 stages) in the middle part (5 to 20 stages from the top of the column), where the concentration of methanol is 0.1 to 3.0 wt% and the concentration of formaldehyde is 0.1 to 1.0 wt% An amount equivalent to the amount of water to be added is withdrawn as a sidestream, and this is added to the raw material gas and reacted. Therefore, the amount of water added from the top of the absorption tower is 2 to 5 times that of the usual method, and accordingly, the content of formaldehyde, methanol, methyl formate, etc. in the waste gas discharged from the top of the absorption tower is remarkable. Decrease. Further, the side stream extracted from the upper tray column contains 1 to 4 times, especially 2 to 3 times by weight, methanol of formaldehyde, and the addition of this to the raw material gas improves the methanol unit consumption. And methanol is extracted as a sidestream, the amount of methanol mixed in the product formaldehyde aqueous solution is reduced, and it is possible to obtain a formaldehyde solution having a low methanol content despite the reaction under methanol-rich reaction conditions. .
The product formaldehyde solution obtained by the present invention has a formaldehyde concentration of 37 to 60% by weight, preferably 45 to
60 wt%, methanol concentration 0.5-3.0 wt%, preferably 0.5-1.0 wt%, the amount of formic acid by-produced at this time is 100 wtppm or less, usually about 50-60 wtppm Is. The water extracted as a sidestream is heated to 60 to 80 ° C. by a heat exchanger and is contacted with the raw material air to evaporate to form a raw material gas.
6) Effect of the Invention According to the present invention, the amount of absorbed water introduced from the top of the absorption tower is larger than that in the usual method, so that unabsorbed components in the waste gas are reduced,
Not only can the unit consumption of methanol be improved by that much, but it is also possible to react under mild reaction conditions by adopting methanol-rich reaction conditions on the premise of recovery of residual methanol. This also leads to the advantage that the amount of water vapor to be mixed can be reduced, and the effect is extremely large.
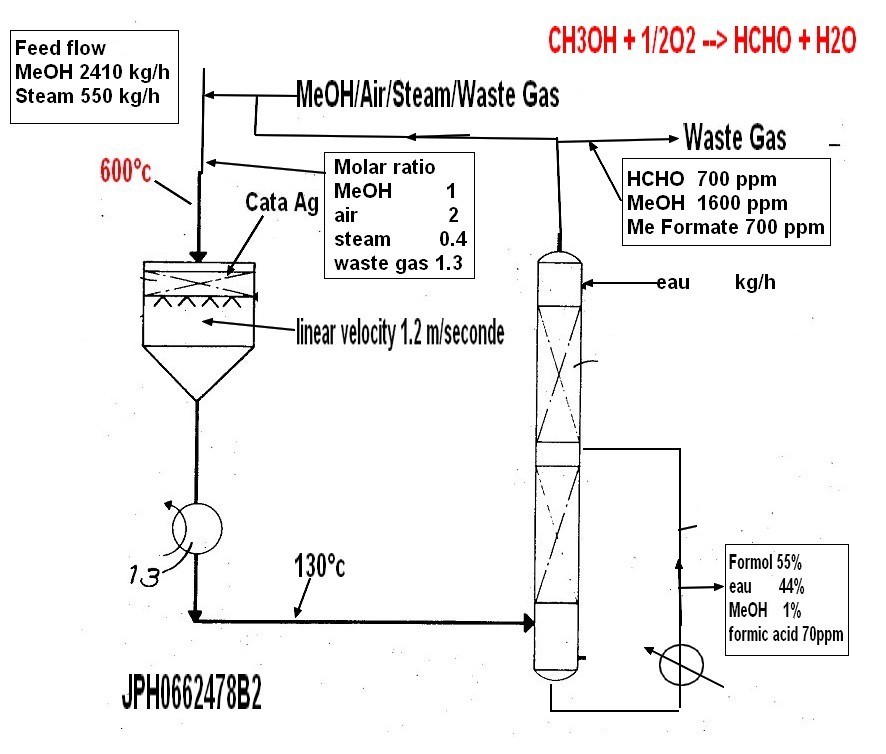
7) Example Example-1 In FIG. 1, 2410 kg / hr of methanol was introduced into 1 from FIG. Also from 3 air 3050 Nm 3 / hr
Is introduced into the humidifier 4 and extracted from 5 and the heat exchanger 6
Countercurrent contact with 600 kg / hr of flowing water on the absorption tower side heated to ℃. The sidestream water contained 2% by weight of methanol. Humidified water is extracted from the bottom of the tower, and the temperature is 65 ° C and 80%.
It was circulated at a flow rate of m 3 / hr. Air temperature at the humidifier outlet is 63
C., and the amount of humidification is adjusted by keeping this temperature constant. The heated methanol vapor and air are mixed and further enter reactor 8 with the waste gas from 7. The molar ratio of methanol: air: steam: waste gas at the reactor inlet is 1: 1.8: 0.4: 1.3. A silver catalyst is held in the reactor and reacted at a reaction temperature of 620 ° C. to form formaldehyde. The reaction product gas exchanges heat with pressurized water by a waste heat boiler attached to the lower part of the reactor and is cooled to 120 ° C, then enters the bottom of the series column lower packed column 9 and countercurrently flows with circulating water at 60 ° C of 10 140 m 3 / hr. Contact. Most of the formaldehyde and part of the methanol are absorbed here, and the residual gas is further circulated in the middle packed column 11 at a temperature of 30 ° C 12 140 m 3
In countercurrent contact with / hr, almost all of formaldehyde is absorbed. Further, the unabsorbed gas is led to the upper tray tower 13,
In countercurrent contact with 700 kg / hr of absorbed water having a temperature of 20 ° C. introduced from the top, methanol and formaldehyde are completely absorbed. The input gas extracted from the top of the tower is formaldehyde 100ppm, methanol 300ppm, methyl formate 400.
It only contained ppm. The product formaldehyde solution was obtained at a rate of 3650 kg / hr from 15, the formaldehyde concentration was 55% by weight, and the methanol concentration was 1.
It was 0% by weight and formic acid content was 50 ppm by weight.
Example-2 As shown in FIG. 2, the waste gas 7 was introduced into the humidifier 4 together with air to increase the amount of humidification. The composition of the raw material gas obtained as a result is methanol: air: steam: waste gas = 1: 1.8: 0.6:
1.3.
The reactor temperature was maintained at 610 ° C. The operating conditions of the absorption tower are almost the same as in Example 1, but the amount of absorbed water from the top of the absorption tower is 1000 kg / hr, and the side-stream withdrawal amount is 900 kg.
/ Hr. The product formaldehyde solution was obtained at the rate of 3950 kg / hr, the formaldehyde concentration was 51% by weight, the methanol was 0.7% by weight, the formic acid content was 50% by weight, and the waste gas contained 80% formaldehyde and 25% methanol.
It contained 0 ppm and 350 ppm of methyl formate.
Example-3 In FIG. 1, 2250 kg / hr of methanol was introduced into the evaporator 2 from 1 and made into a gaseous state. In addition, air from 3 is 2530 Nm 3 / hr
Is introduced into the humidifier 4 and extracted from 5 and the heat exchanger 6
The countercurrent contact of 900 kg / hr of flowing water on the absorption tower side heated to ℃. This sidestream water contained 1.7% by weight of methanol. The water from the humidifier is withdrawn from the bottom of the tower and the temperature is 65 ° C.
Circulation was carried out at a flow rate of 0 m 3 / hr. Air temperature at the humidifier outlet is 6
It is 2 ° C., and the amount of humidification is adjusted by keeping this temperature constant. The heated methanol vapor and air are mixed and enter the reactor 8 (waste gas is not mixed).
The molar ratio of methanol: air: steam at the reactor inlet is 1: 1.6: 0.7. A silver catalyst is held in the reactor and reacted at a reaction temperature of 620 ° C. to form formaldehyde. The reaction product gas exchanges heat with pressurized water in a waste heat boiler attached to the lower part of the reactor, is cooled to 110 ° C., and then enters the bottom part of the absorption tower lower packed column 9, and the circulating water at 60 ° C. 10 1
Countercurrent contact with 40 m 3 / hr. Most of the formaldehyde and part of the methanol are absorbed here, and the residual gas is countercurrently contacted with the circulating water of 12 140 m 3 / hr at 30 ° C in the middle packed column 11 and almost all of the formaldehyde is absorbed. .
Further, the unabsorbed gas is introduced into the upper tray column 13 and comes into countercurrent contact with 1400 kg / hr of absorbed water having a temperature of 20 ° C. introduced from the top, and methanol and formaldehyde are completely absorbed. The waste gas extracted from the top of the tower is formaldehyde 6
It contained only 0 ppm, 200 ppm methanol, and 300 ppm methyl formate. The product formaldehyde solution was obtained from 15 at a rate of 3890 kg / hr, the formaldehyde concentration was 47.6% by weight, and the methanol concentration was 3.4.
% By weight, and the formic acid content was 50 ppm by weight.
Comparative Example 1 In FIG. 1, there is no side stream 5 and humidifier 4, and the steam added to the raw material gas was mixed with other raw material gas (methanol, air, waste gas) in front of the reactor 8.
That is, 2410 kg / hr of methanol is introduced from 1 into the evaporator 2 and made into a gaseous state. In addition, 3 3 Nm 3 / hr of air was introduced from 3, and the heated methanol vapor and air were mixed,
Add 550 kg / hr of steam. The mixed gas also enters reactor 8 along with waste gas from 7.
The molar ratio of methanol: air: steam: waste gas at the reactor inlet is 1: 2.0: 0.4: 1.3.
A silver catalyst is retained in the reactor and reacts at a reaction rate of 650 ° C to form formaldehyde. The reaction product gas exchanges heat with pressurized water in a waste heat boiler attached to the bottom of the reactor and is cooled to 130 ° C, then enters the bottom of the absorption tower lower packed tower 9
Countercurrent contact with circulating water 10 (140m 3 / hr) at ℃. Most of the formaldehyde and a part of the methanol are absorbed here, and the residual gas is brought into countercurrent contact with the circulating water 12 (140 m 3 / hr) at 30 ° C in the middle packed column 11, and the unabsorbed gas is further absorbed in the upper plate column. Led to 13,
Most of the formaldehyde is absorbed by making countercurrent contact with 100 kg / hr of absorbed water at a temperature of 20 ° C, which is introduced from the top.
The waste gas extracted from the top of the tower is formaldehyde 700
ppm, methanol 1600 ppm, methyl formate 700 ppm. 3610 kg / hr of product formaldehyde solution was obtained, and the product formaldehyde concentration was 55% by weight.
The methanol concentration was 1.0% by weight, and the formic acid content was 70 ppm by weight.
COMPARATIVE EXAMPLE 2 In Comparative Example 1, 3050 Nm 3 / hr of air was introduced from 3 . The molar ratio of methanol: air: steam: waste gas at the reactor inlet is 1: 1.8: 0.4: 1.3, and the reaction temperature in the reactor is 630 ° C.
The waste gas extracted from the top of the tower is formaldehyde 670
ppm, methanol 1700 ppm, methyl formate 720 ppm. A product formaldehyde solution of 3600 kg / hr was obtained, where the product formaldehyde concentration was 54.6% by weight, the methanol concentration was 1.2% by weight, and the formic acid content was 65%.
It was ppm by weight.
[Brief description of drawings]
Patent Citations (5)
Cited By (10)
Abstract
Lead-silver catalysts have been found to be useful in the production of formaldehyde by oxidative dehydrogenation of methanol vapor with an oxygen-containing gas at elevated temperatures.
BACKGROUND OF THE INVENTION
Silver catalysts in the form of granules, gauze, wire turnings, crystals, and the like have been used for many years to produce formaldehyde by the oxidative dehydrogenation of methanol. Silver crystals are especially suitable for this purpose, since they are very selective and have little tendency to promote side reactions and the formation of by-products under reaction conditions which permit high overall yields.
Numerous prior art patents and publications describe the addition of small amounts of promoters to silver catalysts used in the production of formaldehyde from methanol. For example, U.S. Pat. No. 1,937,381, issued to Bond et al on Nov. 28, 1933 and assigned to du Pont, describes silver crystal oxidation catalysts containing promoters such as oxides of tungsten, vanadium, cerium, thorium, molybdenum, chromium, aluminum and zinc. Silver-cadmium alloy catalysts for the oxidative dehydrogenation of methanol are shown in U.S. Pat. No. 3,334,143, issued to Stiles on Aug. 1, 1960 and also assigned to du Pont. Silver containing up to 10% of an oxide of barium, strontium or calcium and up to 8% of an oxide of indium are taught as methanol oxidative dehydrogenation catalysts in U.S. Pat. No. 4,045,369, issued to Cantaluppi on Aug. 30, 1977 and assigned to S.A.E.S. Getters S.p.A. None of these patents suggest the use of lead in combination with silver as a methanol oxidative dehydrogenation catalyst.
Two literature articles from the Soviet Union, each entitled "Catalytic Properties of Silver Alloys in the Conversion of Methanol into Formaldehyde", also disclose various additives alloyed with silver for the oxidation of methanol to formaldehyde. The first is an abstract of The Russian Journal of Physical Chemistry, 45 (10), 1971 p. 1524. It discloses as methanol oxidative dehydrogenation catalysts silver alloys of aluminum, magnesium, copper, zinc, gallium, germanium, selenium, cadmium, indium, tin, antimony, tellurium and bismuth. The other publication is an article from Tekh. Progr. Dostizh Nauki Khim, Prom. 1973, p. 191-5 abstracted in Chemical Abstracts Vol. 81, 68971, 1974. It describes the same silver alloys as the first abstract except that the selenium and antimony alloys are not included. There is no mention of lead-silver catalysts in these articles. Of specific interest, however, is the description of the attempt to improve silver catalysts by adding tin and germanium (or the same group in the Periodic Table as lead) to improve the oxidative dehydrogenation of methanol to formaldehyde. Both of these additives to silver gave exceptionally poor results for formaldehyde production, compared to the use of silver alone.
A paper by Schwab entitled "Metal Electrons and Catalysts" in the The Transactions of the Faraday Society Vol. 42, 1946 p. 689-697, describes heterogeneous and homogeneous alloys of lead-silver, among other alloys. However, these catalysts were used to dehydrogenate formic acid to hydrogen and carbon dioxide, a reaction substantially different from the oxidative dehydrogenation of methanol to formaldehyde.
Another prior art patent, U.S. Pat. No. 3,948,997, issued Apr. 6, 1976 to Howe et al, describes a process for the vapor phase oxidation of α,β-diols such as ethylene glycol to α,β-diones such as glyoxal at elevated temperatures in the presence of a catalyst containing as essential constituents, one or more metals of Group Ib of the Periodic Table comprising copper, silver and gold, and one or more elements from Group IVa, comprising germanium, tin and lead, and Group Va, comprising nitrogen, phosphorus, arsenic, antimony and bismuth. However, there is no specific disclosure in this patent of a lead-silver catalyst, per se, nor is there any suggestion that any of the catalysts disclosed generically or specifically, could be used for the oxidative dehydrogenation of methanol to formaldehyde.
DETAILED DESCRIPTION OF THE INVENTION
The lead-silver catalysts used in the process of the present invention are readily prepared by impregnating lead onto silver. Preferably, the silver base used is silver crystals which are somewhat porous or irregular, e.g., silver crystals having particle sizes ranging from about 8 to about 40 mesh U.S. screen size (i.e. particles which will pass through a 8 mesh screen but which will be retained on a 40 mesh screen). A small amount of silver crystals having a mesh size larger than 8 mesh, i.e., up to about 10 weight percent of the total crystals, can be tolerated in the methanol conversion, but if the amount of large silver particles becomes excessive, the contact of methanol and oxygen with the silver crystals will be significantly decreased, resulting in lessened formaldehyde production. On the other hand, a small amount of silver crystals smaller than 40 mesh (again up to about 10 weight percent of the total catalyst) can be used in the methanol reaction. However, if a significant amount of smaller particles are present, an undesirable increase in the pressure drop across the catalyst bed may be observed. Particularly preferred are silver crystals having particle sizes ranging from about 20 to about 30 mesh (U.S. screen size).
One technique for preparing the catalysts of this invention involves impregnating silver crystals with a decomposable salt of lead, such as lead acetate, lead nitrate, or the like, and then decomposing the lead salt, for example by heat. Lead can also be applied to silver crystals by immersing the crystals in an organic or aqueous solution of a soluble lead salt and evaporating the solvent, preferably while stirring to obtain a more uniform distribution of lead on the silver crystals. Also a suspension or colloidal solution of a lead compound, prepared by any of the ordinary methods, may be flocculated while in contact with silver crystals to give a lead-silver catalyst.
The catalysts used in the process of this invention comprise silver and lead. The amount of lead in the catalyst can range from about 100 to about 150,000 parts per million, based on the total catalyst, and silver can comprise the remainder of the catalyst. The weight ratio of lead to silver on this basis can range from about 0.0001 to about 0.15. The preferred amount of lead in these catalysts can range from about 1000 to about 7000 parts per million, based on the total catalyst, with silver comprising the remainder of the catalyst. The preferred weight ratio of lead to silver can range from about 0.001 to about 0.007.
Lead-silver catalyst can also be prepared by depositing lead and silver on selected catalyst supports, such as silicon carbide or α-alumina, which do not detrimentally affect oxidative dehydrogenation of methanol to formaldehyde under the reaction conditions of the present invention. Such catalysts and processing conditions are described in copending application U.S. Ser. No. 287,385, filed July 27, 1981 in the names of H. R. Gerberich and E. T. Smith assigned to Celanese Corporation and filed concurrently with this application.
The process of this invention can be carried out in any conventional single stage or multiple stage methanol oxidation reactor at elevated temperatures. Individual reactors require facilities to hold a sufficient amount of oxidative dehydrogenation catalyst and to permit the methanol-oxygen mixture to pass over the catalyst to accomplish oxidative dehydrogenation. Downstream facilities to recover the formaldehyde product, normally in aqueous solution, are also required.
In carrying out the process of this invention, a feed mix of methanol and an oxygen-containing stream, such as pure oxygen, a mixture of oxygen and nitrogen, air, or other oxygen sources, i.e., one containing about 5 mol percent to about 100 mol percent of oxygen, is passed into a reactor containing catalyst comprising lead and silver and reacted at a temperature in the range from about 500° C. to about 700° C., preferably about 550° C. to about 650° C. Diluents such as steam and nitrogen, if desired, can be added to the methanol-oxygen mixture in amounts ranging from about 0.1 to about 10 mole of diluent per mole methanol in the feed, and preferably from about 0.75 to about 3 mole of dilute per mole methanol in the feed. The mole ratio of oxygen to methanol in the feed can range from about 0.15 to about 0.8, and preferably from about 0.2 to about 0.5. The space velocity of the feed entering the reactor generally will be maintained at from about 10 to about 150 reciprocal seconds, and preferably at from about 25 to about 80 reciprocal seconds.
Single stage operations, such as described above, although quite widely used to produce formaldehyde, suffer from the disadvantage that rather high amounts of unconverted methanol are contained in the product emerging from the catalyst bed. The presence of methanol in the exiting formaldehyde solution is undesirable since the methanol, generally, must be separated from the formaldehyde using expensive distillation facilities. However, the need for a separation step can be avoided by using a second oxidation stage of reaction. When carrying out a second oxidation, the effluent gases from the first stage reactor can be cooled, preferably below about 250° C., and mixed with an additional oxygen-containing stream, such as air. The use of such a two stage oxidation process is described in U.S. Pat. Nos. 2,462,413; 3,959,383; 3,987,107 and 4,076,754, among others. The effluent gas-oxygen mixture is then passed through a second stage catalytic oxidative dehydrogenation reactor containing sufficient catalyst to convert substantially all of the remaining unreacted methanol to formaldehyde. Temperatures in the range from about 550° C. to about 700° C., and preferably from about 600° C. to about 675° C., generally will be employed, while the space velocity of the gas in the second stage oxidation reactor generally will be maintained in the range of from about 10 to about 200 reciprocal seconds and preferably from about 30 to about 100 reciprocal seconds.
Any catalyst for the conversion of methanol to formaldehyde, and preferably a silver-containing catalyst, can be used in the second oxidation stage. Lead and silver catalysts, such as those used in the first stage oxidation reaction described above, can be used in the second stage as well. Conventionally used silver crystals, with or without other additives, can also be used.
The effluent gas emerging from the second stage can be passed through a cooler and then passed into the base of an absorber column. A stream of water may be introduced at the top of the absorber column in order to adjust the formaldehyde concentration in the aqueous formaldehyde product which is removed from the bottom of the column. The non-condensable gases entering the absorber are vented at the top of the column to an incinerator or to the atmosphere.
The advantages of the invention can be seen by reference to the following examples
EXAMPLE 1
The unpromoted silver used in this and the following examples as a catalyst per se, as well as a base for the promoted catalysts of the present invention, was 20 to 30 U.S. screen mesh size silver crystals, electrolytic grade, 99.9% purity. To produce the promoted catalysts, an appropriate amount of lead acetate, antimony di-tartrate, or bismuth nitrate was impregnated on the silver crystals.
This was accomplished by first adding a solution of the desired metal salt to the silver crystals using an amount of solution just sufficient to cover the crystals. The liquid was then removed under vacuum, as a vapor, by heating. As an illustration, to prepare a silver catalyst containing 10,000 parts per million lead, lead acetate [Pb(OAc)2.3H2 O)] in the amount of 0.46 gram was dissolved in demineralized water to make 5.8 ml of solution. This solution was poured onto 25 grams of silver crystals. The water solvent was then removed under vacuum at 100° C.
In preparing the antimony-containing catalysts, antimony di-tartrate, which is soluble in demineralized water, was poured on silver catalysts and the aqueous solvent removed by vaporization under vacuum at 100° C. In preparing the bismuth-containing catalysts, bismuth nitrate, dissolved in methanol containing a minor amount of concentrated nitric acid added to aid in the solubilization, was poured on silver crystals and the excess methanol removed by vaporization under vacuum at 100° C. After the catalysts were prepared, they were placed in the catalyst bed of the methanol oxidation unit.
EXAMPLES 2-16
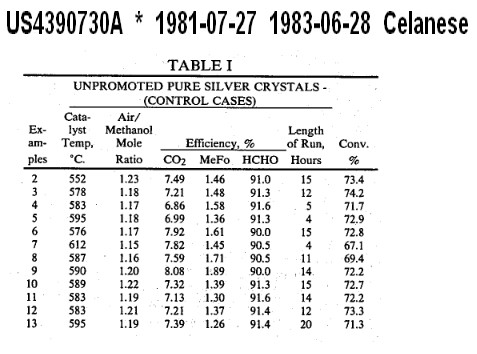
The methanol oxidation unit used for these examples was an insulated, cylindrical reactor made of 316 stainless steel which is 7 inches long and has an internal diameter of 7/8 inch. In each run the reactor contained 17 grams of unpromoted silver catalyst. The catalyst bed was 0.5 inch deep. A thermocouple inserted into the catalyst bed was used to measure the reaction temperature. Air [7605 cc (STP)/min] was sparged into a heated vessel of liquid methanol. The gas leaving this vaporizer contains air to methanol mole ratio of 1.15 to 1.24. This vapor stream was mixed with a flow of pure nitrogen (1949 cc/min) and heated to 125° C. to avoid condensation. The mixture was fed to the methanol oxidation unit described above. Reaction was initiated by heating the catalyst bed with an electrical resistance winding which is on the external surface of the reactor. As soon as the methanol conversion reaction occurred as indicated by a sudden rise in temperature to 450° C. or above the timing of the run was begun. The conversions of methanol, which ranged from about 69 to about 76 percent, were collected by keeping the air and thus the oxygen in the feed stream constant and varying the oxygen-methanol ratio by controlling the methanol in the stream, with higher oxygen-methanol ratios yielding higher methanol conversions. Once the reaction, which is exothermic, was initiated, the temperature was permitted to reach its own level, with no external heat being added to the reaction and no heat being deliberately absorbed by heat exchangers while the reaction is continuing, although an undetermined amount of heat may of course have escaped into the surroundings through the reactor walls. The product stream is analyzed by gas chromatography for mole percent nitrogen, oxygen, methanol, carbon dioxide, carbon monoxide, hydrogen and methyl formate. Data were collected for the period of 5 to 20 hours after initiation of the reaction. The conditions of the reaction were:
catalyst temperature-545°-612° C.
reaction pressure-5.8 psig
oxygen conversion-99.5 percent
space velocity-51 sec-1
Table I sets out the results of the methanol oxidative dehydrogenation to formaldehyde using the unpromoted silver crystals for comparison with the promoted catalysts under the conditions set forth above. The table indicates catalyst temperatures used in the reaction, length of run, conversions of methanol to total products, and the efficiencies of converted methanol to carbon dioxide (CO2), methyl formate (MeFo) and formaldehyde (HCHO).
*************************************************************************************************
Abstract
The invention discloses a production process of paraformaldehyde, which comprises the following steps: comprises a formaldehyde evaporation process, a methanol oxidation process, a formaldehyde absorption process, a formaldehyde concentration process and a paraformaldehyde drying process. The invention belongs to the technical field of paraformaldehyde production, and particularly relates to a paraformaldehyde production process, which is characterized in that methanol and air are used as raw materials, the production process comprises the working procedures of catalytic oxidation, absorption and the like, the methanol is subjected to catalytic dehydrogenation to obtain formaldehyde, the formaldehyde is absorbed by water to obtain a 37% formaldehyde solution, the formaldehyde solution is concentrated, and a vacuum rake dryer is used for drying and producing the formaldehyde solution to obtain a paraformaldehyde finished product.
Description
Paraformaldehyde production process
Technical Field
The invention belongs to the technical field of paraformaldehyde production, and particularly relates to a paraformaldehyde production process.
Background
Paraformaldehyde is a product produced by dehydration, concentration, drying and polymerization of an aqueous formaldehyde solution, is a mixture of formaldehyde molecules, namely polyoxymethylene glycol HO (CH2O) nH and the like, and is a linear polymer important in chemical engineering.
Disclosure of Invention
In order to solve the problems, the invention provides a paraformaldehyde production process.
In order to realize the functions, the technical scheme adopted by the invention is as follows: a production process of paraformaldehyde comprises the following steps:
1) a methanol evaporation process: air is sent into an air washing tower by an air fan, the air enters a methanol evaporator after being washed by water, methanol is sent into the methanol evaporator from a tank area by a pump, the methanol feeding flow is interlocked with the air feeding flow, the feeding proportion is controlled, the formaldehyde liquid generated by the No. 1 absorption tower is used as a heat source to exchange heat with the methanol liquid in the methanol evaporator, so that the temperature of the methanol liquid is raised, and then the methanol gas is generated by bubbling through the air;
2) a methanol oxidation step: introducing a certain amount of water vapor of 0.4MPa and 152 ℃ supplied by an oxidizer steam drum at the top of a methanol evaporator, mixing the water vapor with methanol gas, feeding part of tail gas generated by subsequent reaction into the methanol evaporator through a tail gas fan in proportion to air so as to utilize the heat energy of the tail gas to heat the methanol gas, heating the mixed gas consisting of the methanol gas, the air, the steam and the tail gas containing a small amount of methanol gas to 110 ℃ through pyrogen steam, and sending the mixed gas to a subsequent oxidation section;
3) wherein, the methanol oxidation process comprises the following reaction steps:
CH3OH+1/2O2→CH2O+H2O△H=+156.557kJ/mol CH3OH→
CH2O+H2△H=-85.27kJ/mol
side reaction:
CH3OH+O2→CO+2H2O CH3OH+3/2O2→CO2+2H2O CH2O+
1/2O2→HCOOH HCOOH→CO+H2O;
the mixed gas enters an oxidizer at 110 ℃, is subjected to oxidation reaction under the catalysis of an electrolytic silver catalyst at 580-650 ℃ to generate formaldehyde gas, emits a large amount of heat, soft water is introduced into a jacket of the oxidizer to remove the reaction heat, the water is converted into steam, the steam enters a steam pocket of the oxidizer, the steam is supplied for production between the oxidizer and the oxidizer through a steam distributor, the rest of the steam is introduced into a steam pipe network of a plant area, and meanwhile, the formaldehyde gas at the outlet of the oxidizer is cooled to about 180 ℃;
4) a formaldehyde absorption procedure: the formaldehyde gas generated in the methanol oxidation process is sequentially sent into a 1# absorption tower and a 2# absorption tower for water spraying absorption, tail gas is led out from the top of the 2# absorption tower and is sent to a tail gas boiler, fresh water is supplemented from the top of the 2# absorption tower and is in countercurrent contact with synthesis gas to absorb formaldehyde, a dilute formaldehyde solution extracted from the bottom of the 2# absorption tower is pumped into the 1# absorption tower and is in countercurrent contact with the synthesis gas entering the 1# absorption tower, and the formaldehyde solution (with the concentration of about 37%) is extracted from the bottom of the 1# absorption tower and is sent to a formaldehyde intermediate tank for temporary storage.
5) A formaldehyde concentration procedure: conveying 37% of formaldehyde solution from a formaldehyde intermediate tank into a first-effect evaporator, heating to 80 ℃ under negative pressure for evaporation concentration, mixing gas and liquid, keeping the temperature of 80 ℃, feeding the mixture into a first-effect separator, feeding water vapor from the upper side of the first-effect separator into a vacuum absorption tower for absorption, conveying 55% of concentrated formaldehyde at the bottom of the first-effect separator into a concentrated formaldehyde kettle, and keeping the temperature of the concentrated formaldehyde at 80 ℃ by using a 95 ℃ hot water jacket for heat tracing through a pipeline between the first-effect separator and a dryer and tank equipment so as to prevent the concentrated formaldehyde from self-polymerization;
6) and a paraformaldehyde drying procedure:
the formaldehyde polymerization equation is as follows: nHCHO → (CH2O) n (n ═ 8 to 100)
55% concentrated formaldehyde circularly conveyed by a concentrated formaldehyde kettle is conveyed to a vacuum rake dryer, 50% liquid caustic soda is quantitatively mixed in a liquid caustic soda metering tank, and the liquid caustic soda is pumped into the vacuum rake dryer by a liquid caustic soda feeding pump to catalyze formaldehyde polymerization, so that the formaldehyde polymerization speed is accelerated: introducing 151 ℃ steam into a jacket of the vacuum rake dryer to control the reaction temperature of the materials to be 80 ℃, simultaneously vacuumizing to ensure the negative pressure of the equipment, feeding concentrated formaldehyde and liquid caustic soda into the vacuum rake dryer for polymerization and drying, feeding the dried product into a paraformaldehyde packaging bin, packaging, and conveying to a polyformaldehyde shed area for storage and sale;
7) the tail gas burning contains combustible substances such as H2, CH2O, CH3OH, CO and HCOOH in unabsorbed gas (namely tail gas) that comes out from the top of the 2# absorption tower, part of tail gas is recycled, the tail gas is sent into the methanol evaporator through the tail gas fan to participate in the reaction, the rest tail gas is sent into the tail gas boiler to burn, the tail gas burning generates a large amount of heat, soft water is introduced into the coil pipe of the tail gas processor, the soft water enters the tail gas steam pocket after heat exchange, the generated steam is merged into the steam pipe network of the plant area, and the waste gas generated after the tail gas burning is emptied through the exhaust funnel.
Further, the alcohol feeding flow rate and the air feeding flow rate are interlocked in the step 1), and the feeding proportion is controlled, so that the feeding oxygen-alcohol ratio is kept between 0.39 and 0.45.
Further, the ratio of the oxygen alcohol steam ternary gas in the step 2) is as follows: oxygen: methanol: steam 0.39: 1: 0.25-0.45: 1: 5.
the invention adopts the structure to obtain the following beneficial effects: the production process of the paraformaldehyde provided by the invention is simple to operate, compact in mechanism and reasonable in design, and comprises the following steps: comprises a formaldehyde evaporation process, a methanol oxidation process, a formaldehyde absorption process, a formaldehyde concentration process and a paraformaldehyde drying process. Methanol and air are used as raw materials, the production process of the procedures of catalytic oxidation, absorption and the like is carried out, the methanol is subjected to catalytic dehydrogenation to obtain formaldehyde, the formaldehyde is absorbed by water to obtain a 37% formaldehyde solution, and the formaldehyde solution is concentrated and dried by a vacuum rake dryer to obtain a paraformaldehyde finished product.
Detailed Description
Patent Citations (6)
Similar Documents
Priority And Related Applications
********************************************************************************
A kind of method for preparing anhydrous gaseous formaldehyde
Abstract
The invention discloses a kind of method for preparing anhydrous gaseous formaldehyde, methods described sequentially includes:(1) carry out formaldehyde and prepare reaction;(2) formaldehyde in alcohol absorption step (1) is used to prepare the product reacted;And (3) by the product heats in previous step to 125 to 180 DEG C to discharge anhydrous gaseous formaldehyde.By the present invention, anhydrous gaseous formaldehyde can be directly obtained, and technique is simple, flow is short, and more other anhydrous gaseous formaldehyde preparation method energy consumptions are greatly reduced, and avoid the participation of extra water, so as to which wastewater discharge is also greatly decreased.
Background technology
Formaldehyde is a kind of important Organic Ingredients in chemical field, and its property is active, mainly as production agriculture The raw material of the materials such as medicine glyphosate, phenolic resin, Lauxite, polyacetal resin, melamine resin and centre Body, it is widely used in coating and field of pesticides.
The method of industrial production formaldehyde is very ripe at present, uses methanol oxidizing process mostly, i.e., in silver, copper etc. In the presence of metallic catalyst Oxidation of Methanol is to generate the method for formaldehyde, here, in the oxidizing gas of generation All using water come spray-absorption formaldehyde in absorption, so as to finally give 37%-40% formalin.
But required in the production process of many products using anhydrous formaldehyde, such as resinae, special agricultural chemicals Class etc., and demand is larger.Obtain anhydrous formaldehyde, it is necessary to which presently commercially available formalin is entered Row distillation operation, this will not only put into more considerable equipment, operating cost, and formaldehyde can also be with water shape Into azeotropic mixture, the difficulty of separation is further increased.Or, it is desirable to it can also be adopted in anhydrous reaction With paraformaldehyde, still, the preparation process of paraformaldehyde is also required to formalin being concentrated by evaporation, dried Deng can equally consume substantial amounts of energy, this is not economic and environment-friendly enough.It also proposed in recent years in catalyst In the presence of make methanol direct dehydrogenation generation formaldehyde and hydrogen method, the method reaction after do not generate water, can Directly obtain anhydrous formaldehyde.However, the problem of methods described is present is catalyst easily poisoning and inactivation, circulation Utilization rate is poor, thus also only resides within laboratory demonstration and improved stage, there is not yet large-scale production Report.
Therefore, according to the current method by producing formaldehyde by methanol oxidation, a kind of new production nothing is developed The technique of water beetle aldehyd
According to another implementation of the invention, the formaldehyde prepares reaction and can also be natural gas oxidizing process Or methanol dehydrogenation method etc..Wherein, the main reaction such as following formula of the natural gas oxidizing process (namely for methane oxidizing process) Shown in 2.
[formula 2]
CH4+O2→H2O+HCHO
Also, in the natural gas oxidizing process, used catalyst is this area catalytically oxidizing natural gas (methane) be made formaldehyde custom catalystses, for example, MoO3/SiO2、V2O5/SiO2、Cr2O3/SiO2、 Fe-Mo-Co、Mo/ZrO2Deng catalyst, but the present invention is not limited to this.It is further, since described natural Product in gas oxidizing process also includes water, so the present invention's prepares the method for anhydrous gaseous formaldehyde in step (2) (3) can also further comprise the absorbent of gained in step (2) being evaporated under reduced pressure between to remove moisture Process.
Wherein, the main reaction of the methanol dehydrogenation method is as shown in following formula 3.
[formula 3]
CH3OH→H2+HCHO
Also, in the methanol dehydrogenation method, used catalyst is this area catalysis methanol direct dehydrogenation So that the custom catalystses of formaldehyde are made, for example, Ag/SiO2-ZnO、Ag/SiO2The catalyst such as-MgO, but The present invention is not limited to this.In addition, although the main reaction of the methanol dehydrogenation method does not produce water, but according to Need, the method for preparing anhydrous gaseous formaldehyde of the invention also can be between step (2) and (3) further Including the absorbent of gained in step (2) is evaporated under reduced pressure with dewatered process.
In the present invention, the product that the formaldehyde prepares reaction be gas phase, and it refers to chemically reacting from passing through Generate the derived gas-phase product mixture for including formaldehyde, the gas-phase product mixture in the reactor of formaldehyde May contain unreacted raw material (such as methanol, oxygen, CO), reaction product (for example, formaldehyde, Water, hydrogen etc.) and other foreign gases and/or side reaction product gas etc., wherein the formaldehyde included can To generate hemiacetal with alcohol in procedure described above (2), so as to be dissolved in alcohol, meanwhile, alcohol is again The good solvent of formaldehyde, thus the alcohol not still condensing agent of formaldehyde, or the solvent of formaldehyde.
Therefore, according to an embodiment of the invention, the alcohol can be selected from saturation unitary, binary, One or more in more polynary aliphatic alcohol and polyethylene glycol, poly- propyl alcohol etc..In addition, it is optional, when When the alcohol is aliphatic alcohol, one or more hydrogen on its alkyl chain part can also be by other substituent institutes Substitution, the substituent can be, for example, methyl, hydroxymethyl, cyclohexyl etc..
Preferably, the alcohol can be one kind or more in cyclohexanol, nonyl alcohol, polyethylene glycol and glycerine Kind.By using alcohol enumerated above, formaldehyde can be quickly dissolved and absorbed.
Preferably, the alcohol can be the polyethylene glycol that weight average molecular weight is 200 to 400.In addition, formaldehyde Maximum level in the polyethylene glycol that the weight average molecular weight is 200 to 400 is 50wt%, herein, The maximum level refers to the concept of the amount for the whole formaldehyde that can enter in the polyethylene glycol phase, i.e., at this With the amount of whole formaldehyde existing for hemiacetal state and free state in polyethylene glycol.
Wherein, in step (2), the mol ratio that the alcohol prepares the formaldehyde in product with step (1) is 0.1-3:1, preferably 0.5-1.5:1, more preferably 1:1.
Wherein, the temperature conditionss when gas-phase product is absorbed by alcohol are 10-100 DEG C, preferably 30-80 DEG C, More preferably 60 DEG C.At this temperature, alcohol can effectively dissolve the formaldehyde in the gas-phase product and be formed therewith Hemiacetal.
In the present invention, the reactor for implementing the step (2) does not limit particularly, as long as it is can Realize the reactor of the abundant exchange of gas-liquid, the example may include, but be not limited to, spray column etc. all Plate column and packed tower etc..
According to an embodiment of the invention, the pressure of the vacuum distillation between the step (2) and (3) Power condition is 100-10mmHg, preferably 40-10mmHg, more preferably 20mmHg, and the decompression The temperature conditionss of distillation are 40 to 80 DEG C, preferably 50-70 DEG C, more preferably 68 DEG C.In this temperature and pressure Under the conditions of, water that can to greatest extent in removing step (2) in the absorbent of gained, it is ensured that the present invention is most The water content of anhydrous formaldehyde made from end minimizes, simultaneously because the decomposition bar for the hemiacetal that the present invention is formed Part is more than 125 DEG C, therefore will not also cause the decomposition of hemiacetal in alcohol, avoids the loss of formaldehyde.
In the present invention, the reactor for implementing the vacuum distillation does not limit particularly, as long as it is can be real The reactor being now evaporated under reduced pressure, the example may include, but be not limited to, thin film evaporation tower etc. all be applicable Plate column and packed tower.It by the vacuum distillation process according to the present invention, can contain the dehydrate of generation Water reaches 0.001-0.01wt%, preferably 0.001wt%.
According to an embodiment of the invention, the temperature conditionss of the process of step (3) are preferably 130-170 DEG C, More preferably 140-160 DEG C;And pressure condition is normal pressure to 0.5MPa, preferably normal pressure to 0.4MPa, More preferably normal pressure is to 0.2MPa.Under the conditions of this temperature and pressure, no water beetle can be obtained with stability and high efficiency Aldehyde.
In the present invention, the reactor of implementation steps (3) does not limit particularly, as long as it is achievable The reactor of temperature control heating, the example may include, but be not limited to, the tower knot such as plate column and packed tower Structure, while reactor can also be used.
In the yield of the formaldehyde of preparation in accordance with the present invention, i.e. step (3) amount of the formaldehyde of release with It is more than 92% that formaldehyde of the present invention, which prepares the ratio between amount of whole formaldehyde in the gas-phase product reacted,.
[beneficial effect]
Embodiment 1
The preparation that formaldehyde is carried out using methanol oxidizing process is reacted, and the gas that content of formaldehyde is 30% afterwards is from methanol Column overhead discharge is aoxidized, temperature is down to after 60 DEG C, enters spray tower bottom with 330kg/h flow, together When polyethylene glycol (weight average molecular weight 300) with 1000kg/h flows from spray column top spray, so as to It is 16wt% to content of formaldehyde, content water is 5.4wt% formaldehyde absorption thing.The absorbent is through pump afterwards Squeeze into thin film evaporation tower, be dehydrated, contained under conditions of 68 DEG C of temperature, vacuum 25mm mercury column Water is 0.005wt% dehydrate.The dehydrate is passed through pyrolysis tower with 1100kg/h speed, 155 DEG C are pyrolyzed with heating under normal pressure, so as to obtain the formaldehyde gas that water content is 0.3wt%, yield 96%.
Calculation formula:The amount for the formaldehyde that formaldehyde yield=pyrolysis obtains/formaldehyde is prepared in the gas-phase product of reaction The amount * 100% of formaldehyde
Patent Citations (3)
Cited By (7)
Similar Documents
*********************************************************************************************
CH3OH --->HCHO + H2
CN CN105170145A 王峰 中国科学院大连化学物理研究所
Priority 2014-06-20 • Filed 2014-06-20 • Published 2015-12-23
The invention provides a catalyst for preparing anhydrous formaldehyde through anoxic dehydrogenation of methanol. The catalyst is a metal oxide supported precious metal nanoparticle. When the catalyst is applied in a reaction for preparing anhydrous formaldehyde through direct dehydrogenation of …
Abstract
The invention provides a catalyst for preparing anhydrous formaldehyde through anoxic dehydrogenation of methanol. The catalyst is a metal oxide supported precious metal nanoparticle. When the catalyst is applied in a reaction for preparing anhydrous formaldehyde through direct dehydrogenation of methanol, the reaction is carried out at a low temperature, anhydrous formaldehyde is obtained in a high selectivity manner, and the catalyst has high catalytic activity and long service life.
Background technology
Formaldehyde is a kind of important organic basic industrial chemicals, and in macromolecular material, fine chemistry industry, organic synthesis, medicine intermediate synthesis, fine perfumery synthesis, dyestuff etc., have special applications, economic benefit is very remarkable.Along with the rise of the engineering plastics of synthesized high-performance, PARA FORMALDEHYDE PRILLS(91,95) demand is increasing, and PARA FORMALDEHYDE PRILLS(91,95) concentration requirement is day by day harsh.The existence of water have impact on the exploitation of formaldehyde downstream product.When Lauxite, the phenolic resins of synthesized high-performance, concentration of formaldehyde directly has influence on rate of polymerization and the degree of polymerization, affects the performance of product.And industrial formaldehyde is mainly produced by methanol air oxidizing process, formaldehyde water content is up to more than 50%.The production of anhydrous formaldehyde generally adopts rare aldehyde concentration technology and solvent azeotropic dehydration concentration technology, and because the vapour pressure of formalin is very low, easily form azeotropic system, dehydration separating effect is not good, and energy consumption is large, and isolation andpurification anhydrous formaldehyde is very difficult and expensive.On the other hand, in methanol air oxidizing process, the oxidizable formic acid of formaldehyde, etching apparatus.Therefore, in the urgent need to studying new process, directly produce anhydrous formaldehyde to meet the needs in market by methyl alcohol.
Methyl alcohol direct dehydrogenation is a kind of effective ways preparing anhydrous formaldehyde.The series of problems such as the stability of formalin and purification are all easy to solve.At present, more catalyst mainly a series of metal oxide, slaine, molecular sieve and metal is studied.The research of metal oxide catalyst mainly concentrates on CuO/SiO 2and ZnO/SiO 2.Result of study shows, these catalyst all have good activity and selectivity, wherein ZnO/SiO 2catalyst activity is higher, but stability is generally poor.Weisgickl etc. are with Na 0.5li 0.5alO 2for catalyst, can obtain 98% methanol conversion and 75% formaldehyde selective, but its reaction temperature will up to 900 DEG C, and require harsher to reactor, energy consumption is high, uneconomical.Matsumura etc. are with the ZSM-5 molecular sieve of dealuminzation for catalyst, and formaldehyde yield is 22%.Fudan University Dai Weilin and Fan Kangnian etc. have studied loaded Ag catalyst, has good effect in methanol dehydrogenation.Research finds Ag +be activated centre, in course of reaction, be easily reduced into metallic state and inactivation.At present, report methyl alcohol anaerobic dehydrogenation reaction generally carry out under the high temperature conditions, under hot conditions, formaldehyde is selective low, there is the problems such as carbon distribution.Meanwhile, under high temperature and reducing atmosphere, catalyst is easily reduced fast deactivation.Therefore, be badly in need of exploitation and prepare novel low temperature catalyst, suppressing side reaction generation and catalysqt deactivation.
Summary of the invention
The object of this invention is to provide the catalyst that a kind of methyl alcohol anaerobic dehydrogenation prepares anhydrous formaldehyde, under catalyst action, under anaerobic, cryogenic conditions, methyl alcohol direct dehydrogenation, high selectivity obtains anhydrous formaldehyde.
Conventional methanol anaerobic dehydrogenation process, adopts higher reaction temperature, and high temperature easily makes side reaction occur, and reduces the selective of formaldehyde.The present invention adopts the noble metal nano particles catalyst of support type, realizes the methanol dehydrogenation under cryogenic conditions, reduces the generation of side reaction.The active component of catalyst is one or more in Au, Pd, Pt, Ru metal, and the carrier of catalyst is MgO, CeO 2, ZrO 2, TiO 2, Al 2o 3, one or more in CaO.The load capacity of noble metal is 0.2-15wt%, preferred 0.5%-5.0%.Noble metal nano particles particle diameter is 1-5nm.
The preparation process of catalyst with metal nanoparticles loaded is as follows: the metal component soluble-salt aqueous solution of the 1wt% of certain mass and the protective agent PVPK10 of certain mass are dissolved in 40mL deionized water; then, 0.4wt% sodium borohydride or the reduction under PVPK10 protection of the potassium borohydride aqueous solution of certain volume is added under agitation.This solution, in 20mL ethanol, joins in above-mentioned solution by 0.2g support dispersion, stirs 12h, solvent evaporate to dryness, washing 3-5 time, 80 DEG C of dry 12h.Obtained catalyst screening 20-40 order.
Reaction adopts fixed bed quartz tube reactor on-line measuring device, inserts in quartz reactor, use argon gas purge by obtained catalyst, and the air that removing inside retains and impurity, carry out preheating under argon shield; Wherein methyl alcohol volume content is 10%-60%; Reaction temperature is 100-400 DEG C; The mass space velocity of bed is 5-85mLg -1 cats -1; Particular methanol volume content is 35%-55%; Reaction temperature is 200-300 DEG C; The mass space velocity of bed is 20-55mLg -1 cats -1.
Catalyst tool provided by the invention has the following advantages: the catalytic activity of catalyst is high, (is less than 400 DEG C) at relatively low temperature and reaches high conversion ratio and selective.
Detailed description of the invention
Embodiment 1
Weigh 0.83g1wt%HAuCl 43H 2the O aqueous solution and 0.1gPVPK10 are dissolved in 40mL deionized water, then, add 5mL0.4wt%NaBH under agitation 4.20mL is dispersed with 0.2g carrier TiO subsequently 2ethanolic solution join in above-mentioned solution, stir 12h under room temperature condition, solvent evaporate to dryness, wash 3-5 time, 80 DEG C of dry 12h.Catalyst called after: 2wt%-Au/TiO 2(by Au, load capacity is 2wt%), the average-size of Au ion is 2.5nm, and catalyst is numbered 1, and have Electronic Speculum figure to find out, Au nano particle is dispersed on carrier, and particle diameter is about 2nm.
E
Patent Citations (4)
Cited By (7)
Similar Documents
Priority And Related
*****************************************Abstract
The invention relates to a method for preparing anhydrous formaldehyde by methanol dehydrogenation. The method adopts a palladium-indium catalyst (PdIn/SiO) supported by silicon dioxide2) The specific process is as follows: PdIn/SiO2Filling the formed catalyst into a reaction tube, then filling the reaction tube into a fixed bed reactor, injecting methanol by an advection pump under normal pressure, taking inert gas as carrier gas, wherein the methanol feeding ratio is 10-60 vol%, and the methanol feeding speed is 0.10-0.30 mL/(g)cat.Min), reacting at 350-600 ℃, and obtaining the conversion rate of the methanol of 60-80% and the selectivity of the formaldehyde of 65-85% by chromatography. The invention relates to a method for preparing anhydrous formaldehyde by methanol dehydrogenation, which adopts PdIn/SiO2As the catalyst, the catalyst has higher catalytic activity and product selectivity, less by-products, simple preparation process and high thermal stability.
Background
Formaldehyde is an important organic chemical raw material, can be used for producing thermosetting resins such as phenolic resin and melamine resin, and bulk chemicals such as polyformaldehyde, phenolic aldehyde, urotropine and 1, 4-butanediol, and is also a raw material for synthesizing products such as dye, pesticide, disinfectant and adhesive.
At present, the methanol oxidation method is generally adopted industrially to prepare formaldehyde, theoretically, a formaldehyde aqueous solution with the molar ratio of 1:1 is obtained, the vapor pressure of the formaldehyde aqueous solution is low, the formaldehyde and water form an azeotrope, the separation and purification of the formaldehyde are very difficult, and the energy consumption is huge. In recent years, the demand of engineering plastics, urotropine and other medicines with excellent synthesis performance on anhydrous formaldehyde is increasing, and the anhydrous formaldehyde is obtained by removing moisture from industrial formaldehyde aqueous solution at present, so that the direct preparation of the anhydrous formaldehyde is a hot point of research.
The process for preparing formaldehyde by anaerobic dehydrogenation of methanol obtains formaldehyde and byproduct hydrogen, the formaldehyde and the byproduct hydrogen are easy to separate, and the hydrogen is industrial gas with high added value; the process has no water generation, avoids the separation operation of the formaldehyde aqueous solution, and greatly saves the purchase cost and the operation cost of the rectification equipment; the process also avoids the problem that the formic acid generated by the methanol oxidation method corrodes equipment. Therefore, the direct dehydrogenation of methanol to prepare formaldehyde is a process with great industrial prospect.
In recent years, a new anhydrous formaldehyde preparation process with remarkable economic benefit draws high attention at home and abroad, and a great deal of research work is brought forward. Research efforts are currently focused on the development of highly efficient catalysts, including metals and their oxides, alkali metal salts, and molecular sieves, among others. CN102274722A discloses a new type V2O3And a load type V2O3The preparation method shows better activity in the dehydrogenation reaction of methanol. Takagi et al (Takagi K, Morikawa Y, Ikawa T.Chemestryletters, 1985, 14: 527-0Has high selectivity to formaldehyde. CN101961650A discloses a homogeneous coprecipitation method for preparing zirconium-based catalyst and catalyzing methanol to perform anaerobic dehydrogenation, wherein the yield of formaldehyde reaches 60%. Davirin et al (CN1390639A, CN1537673A, CN1544147A) disclose a series of preparation methods of silver-based catalysts for direct dehydrogenation of methanol. The patent CN101147872A takes industrial sodium bicarbonate as a raw material to prepare the anhydrous formaldehyde by catalyzing and preparing the industrial sodium carbonate, and lays a foundation for the industrialization of preparing the formaldehyde by methanol dehydrogenation. Music et al (Music A, Batista J, Levec J. appliedCatalysis A: general, 1997, 165: 115-131) takes a ZSM-5 molecular sieve catalyst as a matrix, and prepares Na-ZSM-5 and Cu-ZSM-5 type molecular sieves by an ion exchange method, thereby obtaining better selectivity in the methanol dehydrogenation reaction. CN105601487A discloses a rare earth complex Ln [ CH ]2(CH2)nR]3·xH2O.yL, the catalyst has higher selectivity to formaldehyde.
Based on the above, the disadvantages of the catalysts currently used for the preparation of anhydrous formaldehyde are: copper particles in the copper-based catalyst are distributed unevenly, are larger, have smaller active surface area and lower activity; the modified molecular sieve catalyst is difficult to regulate and control in acid-base property and has more byproducts; the catalytic activity of conventional carbonates or bicarbonates is relatively inert and the reaction temperature is generally above 700 ℃. The complex catalyst is a homogeneous catalysis process, and the separation energy consumption is large. Therefore, a heterogeneous catalytic system with good stability, high activity and formaldehyde selectivity and mild reaction conditions is developed, and the preparation of anhydrous formaldehyde by methanol dehydrogenation is imperative.
Disclosure of Invention
The invention aims to overcome the defects of the catalyst used for preparing anhydrous formaldehyde at present, such as: the acidity and the alkalinity of the catalyst are difficult to control, and a plurality of byproducts are generated. The patent combines a palladium-indium alloy with higher activity and a nano-silica carrier without a significant acid center to prepare a silica-supported palladium-indium catalyst. The catalyst has the advantages of simple preparation method, high stability, mild reaction conditions and high conversion rate and selectivity.
The invention relates to a preparation scheme of anhydrous formaldehyde, wherein the preparation of the anhydrous formaldehyde adopts PdIn/SiO2As a catalyst, the catalyst was molded and charged into a reaction tube, and then charged into a fixed bed reactor. Under normal pressure, methanol is injected by an advection pump, inert gas is used as carrier gas, the methanol feeding ratio is 10-60 vol%, and the methanol feeding speed is 0.10-0.30 mL/(g)catMin), reacting at 350-600 ℃, and detecting a product on line by gas chromatography.
The PdIn/SiO2A catalyst, wherein the content of palladium is 0.5-5 wt% (by mass), and the content of indium is 0.5-5 wt%. The PdIn/SiO2The catalyst is prepared by a coprecipitation method and an impregnation method.
The PdIn/SiO2In the preparation of the catalyst, the soluble precursor salt of palladium is one or two of palladium nitrate, palladium ammonium nitrate and sodium chloropalladate, and the soluble precursor salt of indium is one or two of indium nitrate and indium chloride. The PdIn/SiO2In the preparation of the catalyst, the soluble precursor salt of palladium is preferably one or two of palladium ammonium nitrate and sodium chloropalladate, and the soluble precursor salt of indium is preferably indium chloride. The filling PdIn/SiO2The thickness of the catalyst bed layer is 5-40 mm, the methanol feeding ratio is 10-60 vol% (volume), and the methanol feeding speed is 0.10-0.30 ml/(g)catMin), the reaction temperature is 350-500 ℃. The inert gas is one or more of nitrogen, argon and helium.
This patent discloses silica supported palladium indium catalysts with higher catalytic activity. The active component palladium can activate C-H bonds of methanol and O-H bonds of the methanol, and the introduction of indium effectively adjusts the electronic structure and spatial distribution of the palladium, inhibits the excessive dehydrogenation of the target product formaldehyde at palladium sites, and obviously improves the selectivity of the formaldehyde. And the palladium-indium has no obvious acid site, so that the side reaction of dimethyl ether and water generated by methanol dehydration catalyzed by acid is avoided while methanol dehydrogenation is catalyzed, and the high-purity anhydrous formaldehyde is favorably obtained. In addition, the catalyst related to the patent adopts nano silicon oxide as a carrier, has large surface area, is beneficial to high dispersion of palladium and indium, is not easy to agglomerate and deactivate, and has no obvious acid center, thereby avoiding side reaction caused by the acid center and further improving the selectivity and stability of preparing anhydrous formaldehyde by methanol dehydrogenation.
The specific implementation mode is as follows:
in order to further explain the present invention in detail, several specific embodiments are given below, but the present invention is not limited to these embodiments.
Example 1
Coprecipitation method for preparing (0.5 wt% Pd-0.5 wt% In)/SiO2Catalyst, operating as follows: weighing 2g of nano SiO2(50nm), dispersing in 20mL of water, adding one eachQuantitative Na2PdCl4And InCl3·4H2O, stirring for 1h, and adjusting the pH value of the suspension to 11 by using 30% ammonia water. Stirring for 1H, centrifuging, washing to neutrality, oven drying at 80 deg.C, and drying at 500 deg.C H2Reducing for 2h to obtain (0.5 wt% Pd-0.5 wt% In)/SiO2A catalyst. 2g of the catalyst was weighed, tabletted, molded and packed into a fixed bed reactor. Under normal pressure, nitrogen gas is used as carrier gas, methanol is injected by an advection pump, the volume ratio of the methanol is 10 vol%, the feeding speed of the methanol is 0.30mL/(gcat. min), the reaction is carried out at 350 ℃, the conversion rate of the methanol is 73% by gas chromatography online detection, and the selectivity of the formaldehyde is 66%.
Patent Citations (4)
Similar Documents
*******************************************************************
Patent Citations (42)
Abstract
-
[0048] In a quartz tubular reaction, a mixture of 2.0 g of a sample catalyst and 2.0 g of 40 - 60 mesh fused alumina, which was previously proved to be inert in the test reaction, was charged. Methanol was vaporized and mixed with nitrogen at 150°C, and the gaseous mixture (molor ratio, CH30H/N2 = 36/65) was passed through at atmospheric presusre at 250 m mol/hr, whereby the dehydrogenation of methanol was effected at 500° to 600°C. However, in Examples 3 and 4, a gaseous mixture (molar ratio, CH30H/N2 = 42/58) was fed at 375.9 m mol/hr at atmospheric pressure. In Example 13, a gaseous mixture (molar ratio, CH3OH/N2 = 21/79) was fed at 439.5 m mol/hr at atmospheric pressure and, in Example 14, the molar ratio of methanol to nitrogen was 17/83 and feeding rate of the gaseous mixture was 553.1 m mol/hr at atmospheric pressure. In Examples 13 and 14, no alumina was used and only the sample catalyst was packed in the reactor. -
[0049] The gas exhausted from the reactor was directly introduced, with a sampler, to a gas chromatography (heat electroconductivity type) in which a 3 m column packed with APS-201 20 % Flusin (manufactured by Gasukuro Kogyo Inc.) and a 2 m column packed with molecular sieve 13X are used, and the concentrations of formaldehyde (HCHO), methyl formate, dimethyl ether (DME),hydrogen (H2), carbon monoxide (CO), methane (CH4), unreacted methanol (CH30H at the exit) and nitrogen were measured. The results are given in Tables 1 and 2. The data of Examples 1 to 14 and Comparative Examples 1 to 8 were those measured at 8 to 12 hours after the reaction temperature reached to the previously determined value and therefore of the steady state. Since almost no dimethyl ether and methyl formate were detected with the gas chromatographic analysis, the corresponding data are omitted from the Table 1, but no data are given in Table 2, with respect to
Patent Citations (8)
Cited By (8)
********************************************************************************************************
also
US4054609A
AbstractPURPOSE:Formaldehyde, having good thermal and aging stabilities, is prepared in high yield by the dehydrogenation of methy alcohol in the presence of the catalyst consisting of Cu, Zn and Se.
Description
The present invention relates to a process for the preparation of formaldehyde by dehydrogenation of methanol. More particularly, it relates to a process for the preparation of formaldehyde by the dehydrogenation of methanol in the presence of copper, zinc and selenium as catalyst components.
Catalytic oxidative dehydrogenation, catalytic oxidation and the like are generally known as a commercially available process for the preparation of formaldehyde. According to these prior art processes, formaldehyde is generally obtained as an aqueous solution whose concentration is found to be at most 40% by weight.
These aqueous formaldehyde solutions are very unstable, and so they are not satisfactory in both quality and handling. For instance, precipitation of paraformaldehyde during storage and transportation, as well as formation of formic acid by a side reaction, may befrequently observed. Further, paraformaldehyde which is obtained by the concentration of the aqueous formaldehyde solution has a defect that its solubility decreases several days after the preparation although the paraformaldehyde obtained just after the preparation is comparatively well soluble in water, an alcohol, etc. in a few days after the preparation.
On the other hand, there have been proposed many methods for the production of formaldehyde by the so-called dehydrogenation of methanol. For example, a method using a catalyst consisting of copper, silver and silicon (see U.S. Pat. No. 2,939,883), a method using a catalyst having metallic zinc adhered on the surface of a metallic copper support (Japanese Patent Publication No. 11,853/1966), a method using fused zinc, gallium, indium or aluminum or an alloy thereof (Japanese Patent Publication No. 19,251/1972), and a method contacting methanol with fused zinc containing carbon or a zinc alloy containing carbon (Japanese Patent Laid Open No. 97,808/1973) have been proposed. However, even these methods have common serious disadvantages in that the life of their catalysts and the conversion obtained are so poor as to make these methods not industrially acceptable.
We also proposed several processes for the preparation of formaldehyde in a high yield by using a novel catalyst: a process wherein a catalyst prepared from copper, zinc and sulfur is used (Japanese Patent Application No. 63,984/1974) and a process wherein sulfur is fed in gaseous state in order to prevent deterioration of the catalyst (Japanese Patent Application No. 148,390/1974). These processes are superior to the prior art processes in that the catalysts have high activity; but the sulfur undesirably entrains into the reaction product or the exit gas in accordance with these process because of using sulfur as the catalyst.
Accordingly, it is an object of the present invention to provide a novel process for the preparation of formaldehyde.
Another object of the present invention is to provide a process for the preparation of formaldehyde by dehydrogenation of methanol by using a catalyst without any sulfur-exhausting problem.
A further object of the present invention is to provide a process for the preparation of formaldehyde which is excellent in both thermal stability and storage stabilility. A still further object of the present invention is to provide an excellent catalyst for the preparation of formaldehyde.
In accordance with the present invention, these objects can be accomplished by a process for the preparation of formaldehyde comprising subjecting methanol to dehydrogenation in the presence of a ternary metal catalyst comprising copper, zinc and selenium.
The atomic ratio of these metals constituting the catalyst of the present invention may be as follows: copper/zinc/selenium 1:0.01-0.5:0.01-0.5, preferably 1:0.1-0.4:0.1-0.4, provided the amount of selenium preferably should not exceed that of zinc.
The catalyst used in the present invention may be prepared by any one of conventional procedures known to those skilled in the art, for example, precipitation method, thermal decomposition method, or deposition and drying method. Any of these procedures may be properly selected based on the raw material to be used.
The raw materials for the catalyst in the present invention include a copper salt of a mineral acid such as copper nitrate, copper chloride, copper sulfate and copper sulfite, etc., copper hydroxide, copper oxide, basic copper cabonate, metallic copper, etc. as a copper source; a zinc salt of a mineral acid such as zinc nitrate, zinc chloride, zinc sulfate and zinc sulfite, etc., zinc hydroxide, zinc oxide, metallic zinc as a zinc source; and selenic acid, selenious acid, selenium oxide, metallic selenium, etc. as a selenium source. Further, zinc selenide, zinc selenate, zinc selenite, etc. may be used as both zinc and selenium sources and copper selenide may be used as both copper and selenium sources.
The raw material for the catalyst may be in the form of either powder or grains, etc.
The above raw materials may be formed to a particle having a desired shape which may be tablet, sphere or the like and the average diameter of the particles thus formed would be more than 1 mm, preferably 2 to 5 mm. The catalyst particles are then reduced in a reductive atmosphere, for example, in two steps, first at a temperature of 100° to 300° C, preferably 150° to 250° C for more than 0.2 hour, preferably 0.5 to 1 hour and then at the temperature of 500°-750° C, preferably 600°-700° C for more than 0.1 hour, preferably 0.5-1 hour.
The reaction temperature for the present invention may be 500° to 750° C, preferably 600° to 700° C in a catalyst bed. If it is below 500° C, conversion decreases, and if it is higher than 750° C, yield decreases. This reaction temperature is a temperature conventionally employed in the art.
The dehydrogenation reaction in the present invention, generally, may be carried out under atmospheric pressure. However, it may be carried out under reduced or superatmospheric pressure, if desired.
Methanol is generally fed in vapor form together with hydrogen gas to the catalyst bed. Methanol may be preferably fed in an amount of 0.2 to 0.7 mol/hr per 20 ml of the catalyst. The feed amount of less than 0.2 mol/hr is not practical, whereas that of more than 0.7 mol/hr shows decreased conversion of methanol.
According to the present invention, the product obtained contains 30 to 85% by weight of formaldehyde, 0 to 2% by weight of water and the balance of methanol. It is to be appreciated that formaldehyde can be obtained in a high yield, with a very small water content.
The product obtained in accordance with the present invention has quite a different property from an aqueous formaldehyde solution or paraformaldehyde prepared by any conventional method. That is to say, when the formaldehyde concentration in the product obtained by the present invention is not more than 70% by weight, the product is a clear liquid at an ordinary temperature and does not show any appreciable solid precipitation, even if it is left alone at an ordinary temperature for a long period of three months or more. When the formaldehyde concentration is more than 70% by weight, the product is a white solid at ambient temperature, but it melts easily by heating to form a clear liquid, and then it becomes solid again on cooling. Even if such melting by heating and soldification by cooling are repeated, the fusion temperature does not change. For example, the product in the present invention containing 73.0% by weight of formaldehyde, 25.9% by weight of methanol and 1.1% by weight of water is solid at ordinary temperature, but it becomes a clear liquid by heating at a temperature of 30° to 35° C and it solidifies again by cooling. Such a heating-cooling cycle is repeated once a day for 30 days, but the melting temperature did not change. This fact shows that the product obtained by the present invention is more stable compared with those obtained by the prior art processes.
The present invention is illustrated in detail by the following examples, in which conversion and yield are defined as follows:
EXAMPLE 1
Each aliquot, 50.0 g of cupric oxide, 7.0 g of selenium dioxide and 7.0 g of zinc oxide, was ground and mixed thoroughly in a kneader. Thereto, 30 ml of water was added to obtain a paste, which was in turn formed into tablets each being 3 mm in diameter and 3 mm in height. The tablets thus obtained were reduced in a hydrogen gas stream at a temperature of 200° C and then at a temperature of 650° C respectively for 30 minutes to obtain a catalyst.
20 ml of the catalyst thus obtained was packed in a quartz glass-made tubular reactor having an inner diameter of 21 mm, and 15.0 g (0.468 mol)/hr of methanol vapor and an equimolar amount of hydrogen gas were fed thereto continuously from one end of the reactor and subjected to reaction at a catalyst bed temperature of 650° C. Conversion of 78.1% and yield of 66.0% were obtained. The catalytic activity was maintained for more than 50 hours. Composition of the product is 73.0% by weight of formaldehyde, 1.1% by weight of water and 25.9% by weight of methanol in the condensed phase and 87.3% by volume of hydrogen, 9.1% by volume of carbon monoxide, 2.7% by volume of methane and 0.9% by weight of carbon dioxide in the gas phase. The gas phase constitutes 15% by weight on total weight basis while the condensed phase comprises 85% by weight.
The amount and composition of the gas phase is nearly the same all in the following examples.
EXAMPLE 2
A catalyst was prepared from 40.0 g of cupric oxide and 14.5 g of zinc selenide as raw materials in the same manner as in EXAMPLE 1. The reaction was carried out by using the catalyst under the same conditions as in EXAMPLE 1 thereby to obtain a conversion of 73.8% and a yield of 64.5%. The catalytic activity was maintained over more than 55 hours. Further, the composition of the product in the condensed phase was 69.3% by weight of formaldehyde, 0.7% by weight of water and 30.0% by weight of methanol.
EXAMPLE 3
A catalyst was prepared from 50.0 g of powdered copper and 60.6 g of zinc selenite as raw materials in the same manner as in EXAMPLE 1. The reaction was carried out under the same conditions as in EXAMPLE 1 to obtain a conversion of 69.0% and yield of 62.1%. The catalytic activity was maintained over more than 50 hours. The composition of the product in the condensed phase was 64.9% by weight of formaldehyde, 0.5% by weight of water and 34.6% by weight of methanol.
EXAMPLE 4
A catalyst was prepared from 50.0 g of cupric hydroxide anhydride and 2.0 g of zinc selenide as raw materials in the same manner as in EXAMPLE 1. The reaction was carried out under the same conditions as in EXAMPLE 1 to obtain a conversion of 65.5% and a yield of 59.4%. The catalytic activity was maintained over more than 35 hours. Composition of the product in the condensed phase was 61.5% by weight of formaldehyde 0.5% by weight of water and 38.0% by weight of methanol.
EXAMPLE 5
Copper nitrate trihydrate (242.0 g) and zinc nitrate hexahydrate (40.7 g) were dissolved in 1500 ml of water to obtain nitrate solution. Separately, sodium hydroxide (91.0 g) was dissolved in 500 ml of water to obtain an aqueous sodium hydroxide solution and the aqueous solution was added dropwise to the nitrate solution over 1 hour. The precipitate thus obtained was separated by filtration and then dispersed into about 1500 ml of water. The resulting dispersion was vigorously stirred for 15 minutes and was filtered with stirring to obtain a cake. Such washing procedure as above was repeated six times. Selenium dioxide (11.1 g) was added to the cake thus obtained and the cake was crushed, mixed, formed and reduced in the same manner as in EXAMPLE 1 to obtain a catalyst.
The reaction was carried at a catalyst bed temperature of 680° C by using the catalyst thus obtained under the same conditions as in EXAMPLE 1 to obtain a conversion of 88.5% and a yield of 57.6%. The catalytic activity was maintained over more than 50 hours. The composition of the product in the condensed phase was formaldehyde (81.4% by weight), water (1.3% by weight) and methanol (17.3% by weight).
COMPARATIVE EXAMPLE 1
A catalyst was prepared in the same manner as in EXAMPLE 1 but without using selenium dioxide. The reaction was carried out using the catalyst under the same conditions as in EXAMPLE 1 to obtain a conversion of 98.2% and a yield of 12.2%. The yield was considerably lower than those of the EXAMPLES.
COMPARATIVE EXAMPLE 2
A catalyst was prepared in the same manner as in EXAMPLE 1 but without using zinc oxide. The reaction was carried out by using the catalyst under the same conditions as in EXAMPLE 1 to obtain a conversion of about 60% and a yield of about 50%, after 1 to 2 hours from the start of the reaction. The catalytic activity, however, deteriorated gradually, and conversion and yield decreased respectively to 40% and 35% after 5 hours from the start.
EXAMPLE 6
EXAMPLE 1 was repeated, except that the reaction was carried out at a catalyst bed temperature of 700° C. in place of the catalyst bed temperature of 650° C., thereby to obtain a conversion of 90.2% and a yield of 55.1%. The catalytic activity was maintained over more than 50 hours. Further, the composition of the product in the condensed phase was 83.6% by weight of formaldehyde, 0.6% by weight of water and 15.8% by weight of methanol.
EXAMPLE 7
EXAMPLE 1 was repeated, except that the reaction was carried out at a catalyst bed temperature of 600° C. in place of the catalyst bed temperature of 650° C., thereby to obtain a conversion of 60.2% and a yield of 55.3%. The catalytic activity was maintained over more than 70 hours. Further, the composition of the product in the condensed phase was 55.5% by weight of formaldehyde, 1.9% by weight of water and 42.6% by weight of methanol.
EXAMPLE 8
A catalyst was prepared from 50.0 g of cupric oxide, 5.0 g of zinc hydroxide and 5.6 g of selenium dioxide as raw materials in the same manner as in EXAMPLE 1. The reaction was carried out by using the catalyst under the same conditions as in EXAMPLE 1 thereby to obtain a conversion of 93.4% and a yield of 49.3%. The catalytic activity was maintained over more than 50 hours. Further, the composition of the product in the condensed phase was 87.2% by weight of formaldehyde, 0.4% by weight of water and 12.4% by weight of methanol.
EXAMPLE 9
A catalyst was prepared from 50.0 g of cupric oxide, 15.0 g of zinc oxide and 14.4 g of selenium powder as raw materials in the same manner as in EXAMPLE 1. The reaction was carried out by using the catalyst under the same conditions as in EXAMPLE 1 thereby to obtain a conversion of 53.7% and a yield of 48.8%. The catalytic activity was maintained over more than 60 hours. Further, the composition of the product in the condensed ph
ase was 48.8% by weight of formaldehyde, 1.9% by weight of water and 49.3% by weight of methanol.
Claims (6)
Hide Dependent
What we claim is:
1. A process for the preparation of formaldehyde, comprising subjecting methanol in the vapor phase to dehydrogenation in the presence of a catalytically effective amount of a catalyst consisting of copper, zinc and selenium as catalyst components at a temperature of 500° to 750° C. wherein the atomic ratio of said catalyst components is 1:0.1-0.5 : 0.01-0.5 for Cu:Zn:Se.
2. A process according to claim 1, wherein the dehydrogenation was carried out at a temperature of 600° to 700° C.
3. A process according to claim 1, wherein the catalyst has the copper/zinc/selenium atomic ratio of 1:0.1-0.4:0.1-0.4.
4. A process according to claim 1, wherein the methanol is fed together with hydrogen gas to a catalyst bed.
5. A process according to claim 1, wherein the methanol is fed to a catalyst bed in an amount of 0.2 to 0.7 mol/hr per 20 ml of the catalyst.
6. A process according to claim 5, wherein the methanol is fed with hydrogen gas, to a catalyst bed.
Patent Citations (2)
Cited By (11)
*****************************************************************************************************************
The invention relates to a process for the production of formaldehyde by the dehydrogenation of methanol in the presence of copper, zinc: and tellurium-containing catalysts at a temperature of 300 to 750 ° C.
It is known from the German Auslegeschrift 11 44 252 and from Ullmanns Encyklopadie der technischen Chemie (4th edition), Volume 11, pages 693 to 696, methanol in the presence of metal oxide catalysts, usually 'on iron oxide and molybdenum oxide without a carrier to formaldehyde to oxidize. A corresponding catalyst, which also contains cobalt oxide, describes the German patent specification 11 02 344. The German Auslegeschrift 10 63 14 l teaches the oxidation in the presence of such catalysts on a support, carbide and / or iron oxide supports being used. Temperatures from 270 to 380 ° C. are specified. All of these processes yield 37 to 55 percent strength by weight, aqueous formaldehyde solutions by means of catalytic oxidation. The storage and transport of such solutions cause difficulties, since paraformaldehyde precipitates and corresponding deposits and blockages occur in the systems, unless stabilizers or increased storage temperatures are used. Elevated storage temperatures favor the formation of formic acid. This process does not produce low-water methanolic formaldehyde.
For these reasons, processes for the production of low-water formaldehyde solutions with copper / silver / silicon have already been established or copper / zinc or zinc / gallium / indium developed as catalysts, aluminum catalysts and zinc catalysts, those described in German Offenlegungsschrift 25 25 174 will. A dehydrogenation of methanol vapor in a mixture with hydrogen and copper is more advantageous,
u J
1 30033/0344
BASF Aktiengesellschaft - / - OZ 0050/0 ^ 274
'Zinc and sulfur as catalyst components at 500 to 75O 0 C viewed. In the German Offenlegungsschrift 26 27 ^ 21, a similar procedure, which uses selenium as a catalyst component instead of sulfur, is described. It is stated that proportions of sulfur in the catalyst are undesirably entrained in the dehydrogenation by the reaction product or by escaping gas. The disadvantage of the process of German Offenlegungsschrift No. 2β 27 421 is that selenium is also entrained during the reaction and the catalyst is destroyed during prolonged operation. A particular disadvantage is that sulfur and selenium, especially in the form of their hydrogen acids, contaminate the end product and cause environmental protection problems in the exhaust gas. The contaminated end product creates corresponding difficulties in its further processing. Overall, additional measures and systems with regard to the processing, cleaning of the end product and the exhaust gas as well as regulation and control of the systems will be necessary.
2Q It has now been found that formaldehyde advantageously obtained by the dehydrogenation of methanol in the presence of a catalyst at elevated temperature when the reaction is carried out in the presence of copper, zinc, and tellurium-containing catalysts at a temperature of 0 C to 750 JJOO
25 will
The implementation can be represented by the following formulas:
30 CH 3 OH> CH 2 O + H 2
In comparison to the known processes, the process according to the invention surprisingly provides a simpler and more economical one Ways formaldehyde in good yield and purity. With regard to the known processes that lead to aqueous
130033/0344
'. ■' ■ '■ ; : ■: j 300AA36
lead r gene formaldehyde solutions obtained low-water, methanol Ί, advantageously 10 to 40 percent strength by weight formaldehyde solutions. The water content is usually less than 1.5 percent by weight, mostly from 0.2 to 0.9 percent by weight, based on the total solution. The catalyst has a significantly longer service life and activity, usually over or equal to 80 operating hours compared to less or the same 10 operating hours in the case of copper / zinc / sulfur and less than or equal to 40 operating hours in the case
^ q Copper / zinc / selenium as a catalyst for comparable sales. The addition of hydrogen gas can surprisingly be saved will. In view of the publications mentioned above, it was not to be expected that tellurium would occur in the reaction is hardly removed from the catalyst. The content of the reaction
] 5 mixture of comparable conversions is usually = 0.5 rag / kg tellurium, 3 to 175 mg / kg S for sulfur-containing and 5 to 8 ^ 0 mg / kg Se for selenium-containing catalysts. Corresponding the process is more reliable and environmentally friendly and saves additional control and cleaning operations, Systems and frequent catalyst changes. All these beneficial results of the invention Process are in particular with regard to J. Falbe and U. Hasserodt, catalysts, surfactants and mineral oil additives (Thieme Verlag, Stuttgart 1978), page 67, surprising, since it is expressly stated there that contacts for oxidation from acrolein to acrylic acid using cobalt, molybdenum and tellurium oxide precisely because of the volatility of the Tellurs have not found any technical application.
Suitable starting materials for the process are pure methanol or technical-grade methanol. Also raw methanol, that usually as described in DAS 12 77 8 ^ 4 and DP 12 35 88I Process purified by treatment with oxidizing agents and / or alkalis can be used.
130033/0344
BASF Aktiengesellschaft -X- OZ 0050/034274
r The catalyst contains the metals preferably in an atomic ratio of 1 Ί copper at 0.01 to 0.5, preferably 0.05 to 0.4 zinc and from 0.001 to 0.3, preferably 0.005 to 0.2 tellurium. An atomic ratio of 0.05 to 0.3 zinc to 1 copper and / or 0.005 to 0.1 tellurium to 1 c
The parts given in the following examples are parts by weight. They relate to the volume parts as Kilograms to liters. Conversion and selectivity are defined in the following form:
converted methanol (mol)
Sales % = χ 100
] q supplied methanol (mol)
formaldehyde formed (mol)
Selectivity % = x. 100
converted methanol (mol)
15 Example 1
a) Preparation of catalyst: 84 parts of copper (II) oxide, 11.8 parts of zinc oxide and 4.2 parts of tellurium dioxide were mixed well with 50 parts ater V / mm dried at 130 0 C and pills of 3 mm diameter and 3 Processed height.
b) Reaction: parts by volume of the catalyst obtained in this way are placed in a tubular quartz glass reactor (0 3.2 cm). 132 parts / hour of methanol are evaporated, continuously introduced into the reactor and reacted at a temperature of 550.degree. The reaction mixture is condensed. 20.2 parts per hour (selectivity 76% of theory) of formaldehyde in addition to 0.5 parts of water are obtained
3Q ser, 79 parts of methanol. The turnover is 26 percent, the proportion of entrained tellurium <0.5 mg / kg reaction mixture.
1 30033/0344 BATH ORIGINAL,
**********************************************************************************************************************
DETAILED DESCRIPTION OF THE INVENTION <Industrial Field of Application> The present invention relates to a method for producing formaldehyde.
More specifically, the present invention relates to a method for producing formaldehyde, which comprises using a specific zinc-silicon composite oxide as a catalyst for producing formaldehyde by dehydrogenating methanol in the substantial absence of oxygen.
<Prior arts and problems to be solved by the invention> Formaldehyde is used as a resin raw material such as polyacetal resin, urea resin and phenol-formaldehyde resin, or a raw material for various industrial chemicals such as pentaerythritol and hexamethylenetetramine. It is an important basic raw material in the chemical industry.
As a method for industrially producing formaldehyde, a method in which methanol is subjected to catalytic oxidative dehydrogenation with a silver catalyst under air flow, or a catalytic oxidation method using a mixed catalyst of iron oxide and molybdenum oxide in place of the silver catalyst is well known.
It is also well known that in such a method, a large amount of water is produced as a by-product in addition to formaldehyde, and formaldehyde is usually absorbed by water and collected, so that the product obtained is an aqueous solution having a concentration of 30 to 50%. ing. However, these methods have a drawback that complicated and expensive equipments, large amounts of steam, power costs, etc. are required for prevention of catalyst deactivation, removal of by-products, etc. Since it is a water-containing product, it is necessary to remove a large amount of water because it is necessary to remove a large amount of water for raw materials in the field of dislike water, which is in great demand in recent years, especially in the field of resins such as polyacetal resin. It was also a problem in consuming.
In order to solve the problems of the catalytic oxidative dehydrogenation method and the catalytic oxidative method, various methods are also available for the so-called catalytic dehydrogenation method, in which formaldehyde is produced by dehydrogenating methanol in the substantial absence of oxygen. Proposed.
For example, as a catalyst, a method using a catalyst composed of
copper, silver and silicon (Japanese Patent Publication No. 41-11853), a
method using molten zinc, gallium, indium, aluminum or the like
(Japanese Patent Publication No. 47-19251) Sho-48-97808), copper,
A method using a catalyst composed of zinc and sulfur or selenium
(JP-A-51-1407, 51-76209, 52-215) and the like have been proposed, all of
which include the yield of formaldehyde, the selectivity and the
catalyst. It was not possible to select the basic performance of the
catalyst such as the life at the same time.
The present inventors have conducted various studies to solve the above-mentioned problems of the catalytic dehydrogenation method of methanol, and already used a metal oxide obtained by calcining a specific zinc salt and / or indium salt as a catalyst, A method of using a catalyst in which a zinc oxide and / or an indium oxide is supported on silica has been proposed (JP-A-60-4147 and 60-6629).
The
present inventors have conducted extensive studies in order to find a
further excellent method for producing formaldehyde, and as a result, if
a catalyst made of a zinc-silicon composite oxide obtained by a
specific formulation is used,
Further, they have found that formaldehyde can be stably produced in a
high yield over a long period of time, and have reached the present
invention.
<Means for Solving Problems> That is, in the present invention, when a formaldehyde is produced by dehydrogenating methanol in the substantial absence of oxygen, a solution of a zinc compound and a solution of an inorganic silicic acid compound are used as catalysts. An industrially excellent method for producing formaldehyde characterized by using a zinc-silicon composite oxide obtained by forming a precipitate by mixing and then subjecting the precipitate to a calcination treatment at a temperature of 500 ° C. or higher. Is provided.
The
present invention is characterized by using a zinc-silicon composite
oxide obtained by the above-mentioned specific formulation as a
catalyst, but the content of zinc contained in the catalyst is usually 5
˜75 wt%, preferably 20 to 60 wt%, and the atomic ratio of silicon to zinc is usually 1/10 to 10/1.
Cited By (4)
Similar Documents
***************************************************************************************************
worked MeOH diluted in Nitrogne
Description
translated from German
The present invention relates to a method for Production of formaldehyde by dehydration of methanol in the presence of a catalyst at elevated temperature.
There are several methods of making formaldehyde known from methanol. In technology, the oxidation of Methanol to formaldehyde is common
CH₃OH + 1/2 O₂ → CH₂O + H₂O
the catalysts containing iron and molybdenum oxide is carried out at 350 ° C to 450 ° C. It is also common the oxidative dehydrogenation of methanol to formaldehyde.
CH₃OH ⇄ CH₂O + H₂
H₂ + 1/2 O₂ ⇄ H₂O
on silver catalysts at 600 to 720 ° C. Both procedures are described in Ullmann's Encycl. the technical Chemistry, Volume 11, pp. 693-694, 4th edition, 1976, Verlag Chemie Weinheim.
For example, a method for producing Formaldehyde from methanol by increased dehydration Known temperature at which the reaction in the presence one containing at least one sodium compound Catalyst at a temperature of 300 ° C to 800 ° C is carried out (DE-A 37 19 055).
Another publication, in which the use of a catalyst containing silver oxide is mentioned, states that care must be taken to ensure the absence of Al 2 O 3 and MgO as an impurity (JP-A 60 089-441 = Derwent Report 85-1 56 891/26).
However, the known catalysts do not allow economical production of formaldehyde by dehydration of methanol. In the known methods, the Yields of formaldehyde less than 70% in all cases and sales, i.e. the ratio of converted methanol added methanol was never greater than 95%.
The invention therefore relates to a method for manufacturing of formaldehyde by dehydration of methanol in the presence a catalyst at elevated temperature, the Implementation at a temperature of 650 to 1050 ° C below Exclusion of oxygen in the presence of aluminum oxide, Alkali aluminate and / or alkaline earth aluminate as a catalyst in a reactor, the inner wall of which is wholly or partly made Alumina is made.
The reaction can be done through the equation
CH₃OH ⇄ CH₂O + H₂
to be discribed.
The process surprisingly supplies formaldehyde good selectivity and yield.
Are suitable for the preparation of the catalyst Aluminum compounds such as the oxides, hydroxides, carbonates, Bicarbonates, oxalates, acetates or nitrates alone or in Mixture with appropriate alkali or alkaline earth compounds. Coming as cations of the alkali and alkaline earth compounds those of the 1st and 2nd main group of the periodic table in Question like lithium, sodium, potassium, rubidium, magnesium, Calcium, strontium and barium, but preferably lithium and sodium or mixtures thereof. The catalyst can used in a technically common form for example in the form of balls or sticks. He can immediately, i.e. in the initial form of the connections are used or previously thermally, for example the air and / or chemically activated. In any Case, the starting compounds at the high Temperatures in the reactor converted into the active form. Anionic groups such as carbonate, bicarbonate, oxalate, acetate or nitrate after treatment at temperatures, which correspond to the reaction temperatures, no longer proven.
The reaction temperatures during the dehydrogenation of the methanol are generally 650 to 1050 ° C, preferably 820 to 950 ° C and especially 850 to 920 ° C. The one in the process prevailing pressure is not critical. The dehydration of the Methanol can with negative pressure, normal pressure or positive pressure, but preferably carried out at a pressure of about 1.2 bar will.
The methanol, which is in gaseous state in the reactor, can as such or with carbon dioxide, if necessary also mixed with an inert gas, preferably nitrogen to be implemented. It can also contain water be added to the reaction mixture. The added Connections have a positive effect on long-term activity of the catalyst, since these substances of deactivation counteract the catalyst. Generally until 1% by weight of water and / or up to 3 mol% of carbon dioxide, in each case based on the metered amount of methanol used.
The process according to the invention can be carried out batchwise, but preferably be carried out continuously. Here 0.1 to 100 kg, preferably 0.5 to 10 kg, of methanol per hour and per kilogram of catalyst.
The measurands given in the examples were as calculated as follows:
A minor formation of carbon monoxide occurred as a side reaction on. When carrying out the process, in the water to Reaction mixture was added were in the product gas small amounts of carbon dioxide and methane were found. In in no case could water, not even that Reaction mixture metered in, detected in the product gas will.
Examples
1) Sodium carbonate and aluminum hydroxyacetate were mixed so that one mole of aluminum was replaced by one mole of sodium. The mixture was calcined in air at 900 ° C for 3 days. The product thus obtained was triturated and pressed into pellets with a diameter of 6 mm. These were crushed and a grain fraction of 2 mm ± 1 mm in diameter was selected. 1.71 g of the catalyst thus obtained with a bulk volume of 2.2 ml were placed in a tube made of Al 2 O 3 with an inner diameter of 12 mm. The metered methanol contained 0.1 percent by weight of water. At a reaction temperature of 900 ° C. and an inlet of 3.97 mol / h (10 mol% of methanol, remainder nitrogen (N 2 )), the conversion of methanol was 100% and the yield of formaldehyde was 69.4%.
2) Lithium carbonate and aluminum nitrate were mixed so that one mole of lithium accounted for one mole of aluminum. The mixture was calcined in air for 1 1/2 days. The catalyst thus obtained was pressed into pellets with a diameter of 6 mm. These were crushed and a grain fraction with a diameter of 1 mm ± 0.5 mm was selected. 0.75 g of this substance with a bulk volume of 1 ml was placed in an Al 2 O 3 tube with an inner diameter of 12 mm. The metered in methanol contained 0.05 percent by weight of water. At a reaction temperature of 850 ° C. and a feed rate of 3.97 mol / h (10 mol% of methanol, balance N 2 ), the conversion of methanol was 49.2% and the yield of formaldehyde was 23.9%.
************************************************************************************************
EP0261867B1 Process for producing formaldehyde
Current Assignee Sumitomo Chemical Co Ltd
Description
-
[0001] The present invention relates to a process for producing formaldehyde. More particularly, it is concerned with an improved process for producing formaldehyde by dehydrogenation of methanol in the substantial absence of oxygen, in which the catalyst is a specific zinc-silicon complex oxide. -
[0002] Formaldehyde is an important basic raw material in the chemical industry. It is used for the production of synthetic resins such as polyacetal resin, urea resin, and phenol-formaldehyde resin; and chemicals such as pentaerythritol and hexamethylenetetramine. -
[0003] Formaldehyde is commercially produced from methanol by the silver-catalyzed oxidation-dehydrogenation in an air flow, or by a catalytic oxidation process which employs a mixture of iron oxide and molybdenum oxide as a catalyst in place of silver.
These processes, however, have some disadvantages. First, they need a complex and expensive equipment to protect the catalyst from deactivation and to remove by-products, and they also consume a large amount of steam and power. Secondly, they produce a large amount of water as a by-product and they produce formaldehyde in the form of 30 - 50% aqueous solution because the resultant formaldehyde is recovered by absorption into water. A large amount of water in the aqueous solution has to be removed in an instance where formaldehyde is used for the production of polyacetal resin, and this leads to a high energy cost. -
[0004] In order to eliminate the disadvantages of the catalytic oxidation-dehydrogenation process and catalytic oxidation process, there have been proposed several catalytic dehydrogenation processes, by which formaldehyde is produced by dehydrogenating methanol in the substantial absence of oxygen.
The catalyst varies from one process to another. For example, in the process disclosed in Japanese Patent Publication No. 11853/1966, the catalyst is composed of copper, silver, and silicon; in the process disclosed in Japanese Patent Publication No. 19251/1972 and Japanese Patent Laid-open No. 97808/1983, the catalyst is molten zinc, gallium, indium, or aluminum; and in the process disclosed in Japanese Patent Laid-open Nos. 1407/1976, 76209/1976, and 215/1977, the catalyst is composed of copper, zinc, and sulfur or selenium. However, none of these catalysts are satisfactory in all of the basic performances required for catalysts such as yield, selectivity and life. -
[0005] In order to address the above-mentioned problems involved in the catalytic dehydrogenation of methanol, the present inventors carried out a series of researches which matured into their previous inventions relating to a process which employs as a catalyst a metal oxide obtained by burning a specific zinc salt and/or indium salt, and a process which employs as a catalyst zinc oxide and/or indium oxide carried on silica. They are disclosed in Japanese Patent Laid-open No. 4147/1985 and Japanese Patent Publication No. 6629/1985. -
[0006] As the result of their continued studies in search of an improved process for the production of formaldehyde, the present inventors have found that a catalyst composed of zinc-silicon complex oxide of specific composition permits the stable production of formaldehyde with high yield over a long period of time. The present invention is based on this finding. -
[0007] It is an object of the present invention to provide an economically advantageous process for producing formaldehyde by dehydrogenating methanol in the substantial absence of oxygen, said process being characterized in that the dehydrogenation is performed by the aid of a catalyst which is a zinc-silicon complex oxide obtained by mixing a solution containing a zinc salt of an inorganic or organic acid with a solution containing an inorganic silicate compound, or by adding urea to a solution containing a zinc salt of an inorganic or organic acid and an organic silicate compound, thereby forming a precipitate, and subsequently baking the precipitate at 500°C or higher. -
[0008] According to the present invention, the catalyst used in the process is a zinc-silicon complex oxide of specific composition. An amount of zinc in the catalyst should be 5 - 75 wt%, preferably 20 - 60 wt%, and an amount of silicon in the catalyst should be in the range defined by the silicon-to-zinc atomic ratio of 1/10 to 10/1. -
[0009] The zinc moiety of the catalyst may be obtained from a variety of zinc salts. They include zinc salts of inorganic acids such as nitrate and sulfate and zinc salts of organic acids such as carboxylates. Preferable among them are zinc nitrate and zinc carboxylate. -
[0010] The silicon moiety of the catalyst may be obtained from a variety of inorganic silicates such as sodium silicate, potassium silicate, and ammonium silicate, and organic silicates exemplified below. Ammonium silicate is most desirable among inorganic silicates because it does not contain any alkali that might remain in the catalyst. -
[0011] -
[0012] Examples of R¹, R², R³, and R⁴ include alkyl such as methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, sec-butyl, pentyl, cyclopentyl, hexyl, and cyclohexyl; alkenyl such as vinyl, propenyl, allyl, isopropenyl, and cyclohexenyl; alkynyl such as ethynyl and 2-propynyl; aryl such as phenyl, tolyl, and xylyl; aralkyl such as benzyl; and organic carbonyl such as formyl, acetyl, and propionyl. -
[0013] Examples of the organic silicates include tetraalkyl silicates such as tetramethoxysilane and tetraethoxysilane and condensates thereof. -
[0014] In the preparation of the catalyst, aqueous solution of the above-mentioned zinc salt and aqueous solution of the above-mentioned inorganic silicate are mixed together to form a precipitate. The mixing may be accomplished by adding the former to the latter, by adding the latter to the former, or by adding the both to each other simultaneously. The precipitation may be accomplished by controlling the pH with an acid (e.g., sulfuric acid and acetic acid) or an alkali (e.g., ammonia and sodium carbonate). The precipitation may be accomplished at any temperature, usually at room temperature. -
[0015] In the case where aqueous solution of zinc salt and aqueous solution of organic silicate are used, precipitation is accomplished by the aid of urea as a precipitant. Any commercial urea suffices. The urea is used in an amount of 1 - 20 mol, preferably 2 - 10 mol, for 1 mol of zinc. -
[0016] Only for the purpose of precipitation, the urea may be replaced by an alkali such as sodium hydroxide, potassium hydroxide, potassium hydrogen carbonate, sodium carbonate, sodium hydrogen carbonate, and ammonia, which is commonly used as a precipitant for metal ions. However, such an alkali precipitant provides a catalyst having a very small activity. However, the urea precipitant provides a catalyst which has not only an extremely large activity but also a longer life compared with any known catalysts. -
[0017] The function of the urea is not fully elucidated yet. The fact that urea provides a catalyst having an extremely large specific surface area (measured by the BET method) suggests that urea causes the formation of zinc hydroxide and the hydrolysis of organic silicate to proceed simultaneously, thereby forming a precipitate of uniform composition. -
[0018] The preparation of the catalyst from an organic silicate is carried out in the following manner. At first, a precursor of the zinc oxide and an organic silicate are dissolved in alcohol or water or a mixture thereof. In the resulting solution is dissolved urea. Subsequently, the solution is heated at 60 - 150°C for 1 - 100 hours to bring about precipitation. -
[0019] The precipitate formed in the above-mentioned manner is filtered off, washed, and dried. It is finally baked in an air stream or an inert gas stream at 500 -1200°C, preferably 600 - 1100°C, and more preferably about 900 - 1000°C. Thus there is obtained a catalyst which is characterized by that very little zinc is lost by reduction during reaction. The baking at about 900 - 1000°C affords a catalyst which maintains the activity for an extremely long period of time, with almost no loss of zinc. Presumably, this is because the baking at that temperature forms crystalline zinc silicate (Zn₂SiO₄). -
[0020] The catalyst obtained as mentioned above is used for the dehydrogenation of methanol which is usually performed in a flowing gas reaction system. In this reaction system, methanol is supplied in a gaseous form to the catalyst layer, which is usually kept at 450 -650°C, preferably 450 - 550°C. The reaction may be carried out under any pressure, usually atmospheric pressure to 10 kg/cm². -
[0021] Feed methanol may be diluted with an inert gas (e.g., nitrogen, methane, carbon dioxide) and/or hydrogen. Methanol should be fed at a rate of 0.1 -10 kg/hr per kg of catalyst, depending on the volume and shape of the reactor. With a feed rate of less than 0.1 kg/hr, the reaction is not practical, and with a feed rate of more than 10 kg/hr, the conversion of methanol is small. -
[0022] The product gas discharged from the reactor is cooled and then introduced to a heat-exchanger type condenser or an absorbing tower for the recovery of formaldehyde. Incidentally, the product gas contains water in a very small amount, say 0.01 mol or less for 1 mol of formaldehyde. This makes the process advantageous for the production of water-free high-purity formaldehyde. In the case where the absorbent is a higher alcohol such as polyethylene glycol, diethylene glycol, and cyclohexanol, formaldehyde is recovered in the form of hemiformal solution of higher alcohol. Upon thermal decomposition, it readily provides high-purity formaldehyde. -
[0023] According to the process of the invention, the catalyst permits the production of formaldehyde in a high yield at a high conversion of methanol. Moreover, blocking of a catalyst and loss of zinc by reduction are so greatly controlled that production of formaldehyde is ensured for a long period of time with high yield. In addition the process of the invention provides formaldehyde containing a very small amount of water which is able to remove easily. The formaldehyde is especially suitable for the production of polyacetal and oil-soluble phenolic resin, in which contamination of water should be avoided. The production of formaldehyde by the process of this invention also affords a large amount of hydrogen (offgas) which can be effectively utilized as a heat source or feedstock. -
[0024] The present invention, owing to the above-mentioned advantages, is also able to apply to the production of aldehydes or ketones through the dehydrogenation of the corresponding alcohols other than methanol, such as ethanol, butanol, isopropanol, and cyclohexanol. -
[0025] The following examples will be helpful to further understand the invention. -
[0026] Solution-A (aqueous solution of ammonium silicate (NH₄)₂SiO₃) was prepared by passing an aqueous solution of sodium silicate (15.3g of Na₂SiO₃·9H₂O in 100 ml of distilled water) at a flow rate of 500 ml/min through a column filled with 400 ml of ion-exchange resin (Duorite C-22) which had previously been ion-exchanged into NH₄-type. -
[0027] Solution-B (aqueous solution of zinc nitrate) was prepared by dissolving 37.2g of zinc nitrate Zn(NO₃)₂·6H₂O in 100 ml of distilled water. -
[0028] The solution-A was added dropwise with thorough stirring over 30 minutes to the solution-B which was heated at 60°C. After the addition, stirring was continued for additional 30 minutes. The resulting white slurry was filtered and the separated solids were washed with water and dried at 150°C for 12 hours. The solids were baked at 340°C for 2 hours in the air and further baked at 600°C for 5 hours in an electric furnace. Thus there was obtained Catalyst-A. -
[0029] Catalyst-A contains 43.1 wt% of zinc and 21.2 wt% of silicon, and has a specific surface area of 222.6 m²/g (measured by the BET method). In addition, Catalyst-A contains amorphous SiO₂ and ZnO according to X-ray diffractometry. -
[0030] A white slurry was prepared according to the same procedure as in the production of Catalyst-A. The slurry was filtered and the separated solids were washed with water and dried at 150°C for 12 hours. Using an electric furnace, the solids were baked at 350°C for 2 hours in the air and further baked at 1000°C for 5 hours. Thus there was obtained Catalyst-B. -
[0031] Catalyst-B contains 43.1 wt% of zinc and 21.2 wt% of silicon, and has a specific surface area of 40.8 m²/g (measured by the BET method). In addition, Catalyst-B contains amorphous Zn₂SiO₄ alone according to X-ray diffractometry. -
[0032] Solution-B (aqueous solution of zinc nitrate) was prepared by dissolving 37.2g of zinc nitrate Zn(NO₃)₂·6H₂O in 100 ml of distilled water. -
[0033] Solution-C (aqueous solution of sodium metasilicate Na₂SiO₃·6H₂O) was prepared by dissolving 15.3g of sodium metasilicate in 100ml of distilled water. -
[0034] The solution-C was added dropwise with thorough stirring over 30 minutes to the solution-B which was heated at 60°C. After the addition, stirring was continued for further 30 minutes. The resulting white slurry was filtered and the separated solids were washed with water and dried at 150°C for 12 hours. Using an electric furnace, the solids were baked at 350°C for 2 hours in the air and further baked at 600°C for 5 hours. Thus there was obtained Catalyst-C. -
[0035] Catalyst-C contains 42.6 wt% of zinc and 21.5 wt% of silicon, and has a specific surface area of 138.0 m²/g (measured by the BET method). In addition, Catalyst-C contains amorphous SiO₂ and ZnO according to X-ray diffractometry. -
[0036] In a mortar, 10g of commercial zinc oxide ZnO and 7.48g of silicon dioxide were thoroughly mixed. Using an electric furnace, the mixture was baked at 350°C for 2 hours in the air and further baked at 1000°C for 5 hours. Thus there was obtained Catalyst-D. -
[0037] Catalyst-D contains 46.0 wt% of zinc and 20.0 wt% of silicon, and has a specific surface area of 91.1 m²/g (measured by the BET method). -
[0038] In a mortar, 20g of commercial zinc oxide ZnO and 7.48g of silicon dioxide were thoroughly mixed. Using an electric furnace, the mixture was baked at 350°C for 2 hours in the air and further baked at 1000°C for 5 hours. Thus there was obtained Catalyst-E. -
[0039] Catalyst-E contains 58.8 wt% of zinc and 12.7 wt% of silicon, and has a specific surface area of 62.5 m²/g (measured by the BET method). -
[0040] A half of Catalyst-D was baked at 1300°C for 3 hours in the air to give Catalyst-F. -
[0041] Catalyst-F contains 46.0 wt% of zinc and 20.0 wt% of silicon, and has a specific surface area of 0.6 m²/g (measured by the BET method). -
[0042] A half of Catalyst-E was further baked at 1300°C for 3 hours in the air to give Catalyst-G. -
[0043] Catalyst-G contains 58.8 wt% of zinc and 12.7 wt% of silicon, and has a specific surface area of 0.3 m²/g (measured by the BET method). -
[0044] Solution-C (aqueous solution of zinc nitrate) was prepared by dissolving 14.6g of zinc nitrate Zn(NO₃)₂·6H₂O in 200 ml of distilled water. To solution-C was added 20g of silicon dioxide. The resulting slurry mixture was thoroughly mixed on a water bath at 80°C for 1 hour, and subsequently dried under reduced pressure in a rotary evaporator. The resulting solids were baked at 350°C for 2 hours in the air and further baked at 600°C for 5 hours in an electric furnace. Thus there was obtained Catalyst-H. -
[0045] Catalyst-H contains 12.9 wt% of zinc and 38.0 wt% of silicon, and has a specific surface area of 75.0 m²/g (measured by the BET method). -
[0046] The same procedure as in the preparation of Catalyst-H was repeated to give Catalyst-I, except that the baking at 600°C for 5 hours was changed to 1000°C for 5 hours. -
[0047] Catalyst-I contains 12.9 wt% of zinc and 38.0 wt% of silicon, and has a specific surface area of 125.0 m²/g (measured by the BET method). -
[0048] Solution-C (aqueous solution of zinc nitrate) was prepared by dissolving 7.4g of zinc nitrate in 200 ml of distilled water. To solution-C was added 25g of silica sol (trade name "Snowtex N", containing 20 wt% of SiO₂). The resulting slurry mixture was thoroughly mixed on a water bath at 80°C for 1 hour, and subsequently dried under reduced pressure in a rotary evaporator. The resulting solids were baked at 350°C for 2 hours in the air and further baked at 600°C for 5 hours in an electric furnace. Thus there was obtained Catalyst-J. -
[0049] Catalyst-J contains 12.9 wt% of zinc and 38.0 wt% of silicon, and has a specific surface area of 135.6 m²/g (measured by the BET method). -
[0050] A half of Catalyst-J was further baked at 1000°C for 3 hours in the air to give Catalyst-K. -
[0051] Catalyst-K contains 12.9 wt% of zinc and 38.0 wt% of silicon, and has a specific surface area of 65.89 m²/g (measured by the BET method). -
[0052] In 100g of ethanol was dissolved 23.93g of zinc nitrate Zn(NO₃)₂·6H₂O. To the resulting solution was added 16.66g of tetraethoxysilane, followed by stirring for 30 minutes at room temperature. To the solution was added urea solution (28.8g of urea in 84.6g of distilled water), followed by stirring at room temperature for 30 minutes. The solution temperature was raised to 80°C with stirring, and stirring was continued at that temperature for 9 hours to give a white slurry liquid. After being filtered and washed with water, the white solids were dried at 150°C for 12 hours. The dried solids were baked at 350°C for 2 hours in the air and further baked at 600°C for 5 hours in an electric furnace. Thus there was otbained Catalyst-L. -
[0053] Catalyst-L contains 36.2 wt% of zinc and 25.5 wt% of silicon, and has a specific surface area of 296.5 m²/g (measured by the BET method). -
[0054] The same procedure as in the preparation of Catalyst-L was repeated to give Catalyst-M, except that the urea solution was replaced by 58.3g of 28 wt% ammonia water which was added dropwise with stirring over 30 minutes. -
[0055] Catalyst-M contains 34.6 wt% of zinc and 24.5 wt% of silicon, and has a specific surface area of 6.6 m²/g (measured by the BET method). -
[0056] In 39.5g of ethanol was dissolved 53.4g of zinc nitrate Zn(NO₃)₂·6H₂O. To the resulting solution were added 37.2g of tetraethoxysilane dissolved in 39.5g of ethanol, followed by stirring for 30 minutes at room temperature. To the solution was added urea solution (61.5g of urea in 150g of distilled water), followed by stirring at room temperature for 30 minutes. The solution temperature was raised to 80°C with stirring, and stirring was continued at that temperature for 6 hours to give a white slurry liquid. After being filtered and washed with water, the white solids were dried at 150°C for 12 hours. The dried solids were baked at 350°C for 2 hours in the air and further baked at 600°C for 1 hour in an electric furnace. Thus there was obtained Catalyst-N. -
[0057] Catalyst-N contains 44.6 wt% of zinc and 19.5 wt% of silicon, and has a specific surface area of 225 m²/g (measured by the BET method). -
[0058] The same procedure as in the preparation of Catalyst-N was repeated to give Catalyst-O, except that the amount of zinc nitrate was 26.7g in place of 53.4g, the amount of urea 30.7g in place of 61.5g, and the amount of distilled water (as a solvent of urea) 170g in place of 150g. -
[0059] Catalyst-O contains 31.3 wt% of zinc and 27.7 wt% of silicon, and has a specific surface area of 371 m²/g (measured by the BET method). -
[0060] In 200 ml of water was dissolved 18.27g of zinc nitrate Zn(NO₃)₂·6H₂O. To the resulting solution was added 25g of silica sol (tradename "Snowtex N" produced by Nissan Kagaku Co., Ltd. containing 20 wt% of SiO₂). The resulting slurry mixture was thoroughly mixed on a water bath at 80°C for 1 hour, and subsequently dried under reduced pressure in a rotary evaporator. The resulting solids were baked at 350°C for 2 hours in the air and further baked at 600°C for 5 hours in an electric furnace Thus there was obtained Catalyst-P. -
[0061] Catalyst-P contains 40.2 wt% of zinc and 23.4 wt% of silicon, and has a specific surface area of 78 m²/g (measured by the BET method). -
[0062] The above-mentioned catalysts A to P were granulated into 24 - 48 mesh particles. They were stored in a desiccator. -
[0063] The specific surface area was measured with "Monosorb" (tradename made by Quantachrome) after dehydration in a nitrogen stream at 200°C for 30 minutes. -
[0064] To evaluate the catalysts prepared as mentioned above, the dehydrogenation of methanol was carried out by passing a methanol-nitrogen mixture (CH₃OH/N₂ = 18/32 in mol), which had previously been vaporized at 150°C, through a tubular quartz reactor, 10 mm in inside diameter, filled with 1.0g of the sample catalyst. The reaction temperature was 520 - 550°C and the flow rate was 550 mmol/hr under atmospheric pressure. -
[0065] A gas from the reactor was subjected, by means of a heat-insulated gas sampler, to a gas chromatography (thermal conductivity detector) equipped with a 6-m column (APS-201, 20% Fulsin T, made by Gasukuro Kogyo Inc.) and a 2-m column (molecular sieve 13x), to effect quantitative analysis of formaldehyde [HCHO], methyl formate, dimethyl ether, hydrogen [H₂] , carbon monoxide [CO], methane [CH₄], unaltered methanol [CH₃OH at the exit] , and nitrogen. The results are shown in Tables 1 and 2. (The gas chromatography detected almost no methyl formate and dimethyl ether.) -
[0066] Conversion rate of methanol, yield of formaldehyde, and selectivity of formaldehyde are calculated according to the following equations.
where [HCHO],[CO], [CH₄], and [DME] represent the rate (mmol/hr) at which the respective components are formed, and [CH₃OH] represents the flow rate (mmol/hr) of unaltered methanol measured at the exit of the reactor tube. 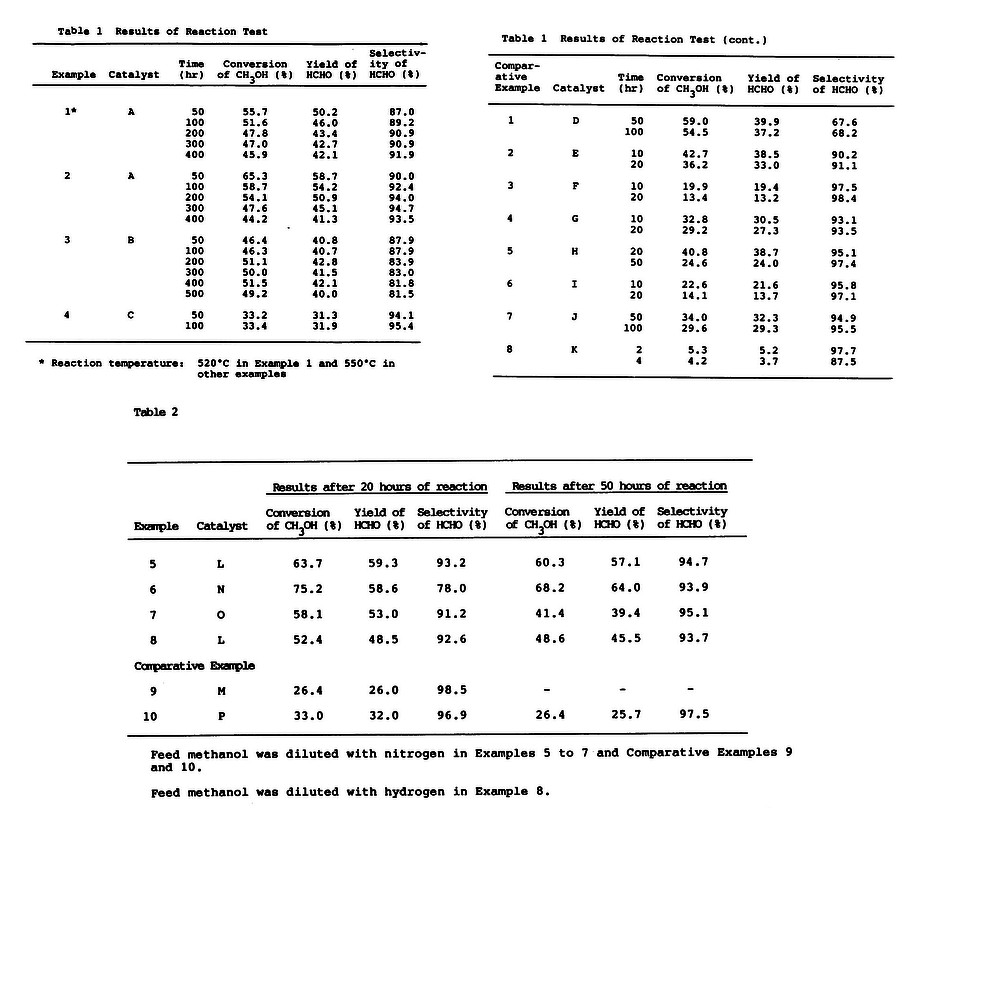
Cited By (4)
********************************************************************************************************************************
*************************************************************************************
*************************************************************************************************************
Abstract
The
invention relates to a method for preparing anhydrous formaldehyde by
methanol dehydrogenation. The method adopts a palladium-indium catalyst
(PdIn/SiO) supported by silicon dioxide2) The specific process is as follows: PdIn/SiO2Filling
the formed catalyst into a reaction tube, then filling the reaction
tube into a fixed bed reactor, injecting methanol by an advection pump
under normal pressure, taking inert gas as carrier gas, wherein the
methanol feeding ratio is 10-60 vol%, and the methanol feeding speed is
0.10-0.30 mL/(g)cat.Min),
reacting at 350-600 ℃, and obtaining the conversion rate of the methanol
of 60-80% and the selectivity of the formaldehyde of 65-85% by
chromatography. The invention relates to a method for preparing
anhydrous formaldehyde by methanol dehydrogenation, which adopts
PdIn/SiO2As the catalyst, the
catalyst has higher catalytic activity and product selectivity, less
by-products, simple preparation process and high thermal stability.
Patent Citations (4)
Similar Documents
Priority And Relate
*********************************************************************************************************************Abstract
The invention relates to a method for preparing anhydrous formaldehyde. The method employs a platinum indium catalyst (PtIn/SiO) supported on silica2) The specific process is as follows: PtIn/SiO2Filling the formed catalyst into a reaction tube, then filling the reaction tube into a fixed bed reactor, injecting methanol by an advection pump under normal pressure, taking inert gas as carrier gas, wherein the methanol feeding ratio is 10-60 vol%, and the methanol feeding speed is 0.10-0.30 mL/(g)cat.Min), reacting at 400-650 ℃, and obtaining the conversion rate of the methanol of 65-90% and the selectivity of the formaldehyde of 60-85% by chromatography. The invention relates to a method for preparing anhydrous formaldehyde by adopting PtIn/SiO2As the catalyst, the catalyst has higher catalytic activity and product selectivity, less by-products, simple preparation process and high thermal stability.
Example 2
Coprecipitation method for preparing (0.5 wt% Pt-1.0 wt% In)/SiO2Catalyst, operating as follows: weighing 2g of nano SiO2(50nm), dispersing in 20mL of water, adding a certain amount of Na2PtCl4And InCl3·4H2O, stirring for 1h, and adjusting the pH value of the suspension to 11 by using 30% ammonia water. Stirring for 1H, centrifuging, washing to neutrality, oven drying at 80 deg.C, and drying at 450 deg.C in H2Reducing for 2h to obtain (0.5 wt% Pt-1.0 wt% In)/SiO2A catalyst. 2g of the catalyst was weighed, tabletted, molded and packed into a fixed bed reactor. Under normal pressure, nitrogen gas is used as carrier gas, methanol is injected by an advection pump, the volume ratio of the methanol is 15 vol%, the feeding speed of the methanol is 0.25mL/(gcat. min), the reaction is carried out at 450 ℃, the conversion rate of the methanol is 77% by gas chromatography online detection, and the selectivity of the formaldehyde is 85%.
*****************************************************************************************************
Abstract
The invention relates to a method for preparing anhydrous formaldehyde by methanol dehydrogenation. The method adopts a palladium-indium catalyst (PdIn/SiO) supported by silicon dioxide2) The specific process is as follows: PdIn/SiO2Filling the formed catalyst into a reaction tube, then filling the reaction tube into a fixed bed reactor, injecting methanol by an advection pump under normal pressure, taking inert gas as carrier gas, wherein the methanol feeding ratio is 10-60 vol%, and the methanol feeding speed is 0.10-0.30 mL/(g)cat.Min), reacting at 350-600 ℃, and obtaining the conversion rate of the methanol of 60-80% and the selectivity of the formaldehyde of 65-85% by chromatography. The invention relates to a method for preparing anhydrous formaldehyde by methanol dehydrogenation, which adopts PdIn/SiO2As the catalyst, the catalyst has higher catalytic activity and product selectivity, less by-products, simple preparation process and high thermal stability.
Background
Formaldehyde is an important organic chemical raw material, can be used for producing thermosetting resins such as phenolic resin and melamine resin, and bulk chemicals such as polyformaldehyde, phenolic aldehyde, urotropine and 1, 4-butanediol, and is also a raw material for synthesizing products such as dye, pesticide, disinfectant and adhesive.
At present, the methanol oxidation method is generally adopted industrially to prepare formaldehyde, theoretically, a formaldehyde aqueous solution with the molar ratio of 1:1 is obtained, the vapor pressure of the formaldehyde aqueous solution is low, the formaldehyde and water form an azeotrope, the separation and purification of the formaldehyde are very difficult, and the energy consumption is huge. In recent years, the demand of engineering plastics, urotropine and other medicines with excellent synthesis performance on anhydrous formaldehyde is increasing, and the anhydrous formaldehyde is obtained by removing moisture from industrial formaldehyde aqueous solution at present, so that the direct preparation of the anhydrous formaldehyde is a hot point of research.
The process for preparing formaldehyde by anaerobic dehydrogenation of methanol obtains formaldehyde and byproduct hydrogen, the formaldehyde and the byproduct hydrogen are easy to separate, and the hydrogen is industrial gas with high added value; the process has no water generation, avoids the separation operation of the formaldehyde aqueous solution, and greatly saves the purchase cost and the operation cost of the rectification equipment; the process also avoids the problem that the formic acid generated by the methanol oxidation method corrodes equipment. Therefore, the direct dehydrogenation of methanol to prepare formaldehyde is a process with great industrial prospect.
In recent years, a new anhydrous formaldehyde preparation process with remarkable economic benefit draws high attention at home and abroad, and a great deal of research work is brought forward. Research efforts are currently focused on the development of highly efficient catalysts, including metals and their oxides, alkali metal salts, and molecular sieves, among others. CN102274722A discloses a new type V2O3And a load type V2O3The preparation method shows better activity in the dehydrogenation reaction of methanol. Takagi et al (Takagi K, Morikawa Y, Ikawa T.Chemestryletters, 1985, 14: 527-0Has high selectivity to formaldehyde. CN101961650A discloses a homogeneous coprecipitation method for preparing zirconium-based catalyst and catalyzing methanol to perform anaerobic dehydrogenation, wherein the yield of formaldehyde reaches 60%. Davirin et al (CN1390639A, CN1537673A, CN1544147A) disclose a series of preparation methods of silver-based catalysts for direct dehydrogenation of methanol. The patent CN101147872A takes industrial sodium bicarbonate as a raw material to prepare the anhydrous formaldehyde by catalyzing and preparing the industrial sodium carbonate, and lays a foundation for the industrialization of preparing the formaldehyde by methanol dehydrogenation. Music et al (Music A, Batista J, Levec J. appliedCatalysis A: general, 1997, 165: 115-131) takes a ZSM-5 molecular sieve catalyst as a matrix, and prepares Na-ZSM-5 and Cu-ZSM-5 type molecular sieves by an ion exchange method, thereby obtaining better selectivity in the methanol dehydrogenation reaction. CN105601487A discloses a rare earth complex Ln [ CH ]2(CH2)nR]3·xH2O.yL, the catalyst has higher selectivity to formaldehyde.
Based on the above, the disadvantages of the catalysts currently used for the preparation of anhydrous formaldehyde are: copper particles in the copper-based catalyst are distributed unevenly, are larger, have smaller active surface area and lower activity; the modified molecular sieve catalyst is difficult to regulate and control in acid-base property and has more byproducts; the catalytic activity of conventional carbonates or bicarbonates is relatively inert and the reaction temperature is generally above 700 ℃. The complex catalyst is a homogeneous catalysis process, and the separation energy consumption is large. Therefore, a heterogeneous catalytic system with good stability, high activity and formaldehyde selectivity and mild reaction conditions is developed, and the preparation of anhydrous formaldehyde by methanol dehydrogenation is imperative.
Disclosure of Invention
Example 3
Coprecipitation method for preparing (1.5 wt% Pd-0.5 wt% In)/SiO2Catalyst, operating as follows: weighing 2g of nano SiO2(50nm), dispersing in 20mL of water, adding a certain amount of Na2PdCl4And InCl3·4H2O, stirring for 1h, and adjusting the pH value of the suspension to 11 by using 30% ammonia water. Stirring for 1H, centrifuging, washing to neutrality, oven drying at 80 deg.C, and drying at 500 deg.C H2Reducing for 2h to obtain (1.5 wt% Pd-0.5 wt% In)/SiO2A catalyst. 2g of the catalyst was weighed, tabletted, molded and packed into a fixed bed reactor. Under normal pressure, nitrogen gas is used as carrier gas, methanol is injected by an advection pump, the volume ratio of the methanol is 30 vol%, AThe alcohol feeding speed is 0.20mL/(gcat. min), the reaction is carried out at 450 ℃, the conversion rate of the methanol is 60 percent by gas chromatography on line detection, and the selectivity of the formaldehyde is 75 percent.
***************************************************************************************************
CN1390639A 2002 Current Assignee Fudan University
Abstract
A carried silver catalyst for preparing anhydrous formaldehyde by directly dehydrogenating methanol is prepared by sol-gel method, which includes such steps as mixing ethyl n-silicate with the solution containing soluble Mg salt, dropping it in aqueous solution of inorganosilver salt, generating colloid, aging, drying and calcining. Its advantages are high activity, long service life and low cost.
Background technology
Formaldehyde is the important foundation raw material of chemical industry, is widely used in the intermediate product of chemical products such as producing acetal resin, Lauxite, phenolic resins, pentaerythrite, methenamine and medicine and agricultural chemicals.And in these fields, most of formaldehyde products of using is anhydrous formaldehyde.As producing anhydrous formaldehyde, then need moisture commodity formaldehyde is carried out distillation operation.This not only needs to drop into comparatively considerable equipment, operating cost, but also, cause forming azeotropic system, thereby in actual distillation operation process because formalin relative ideal solution is the minus deviation of certain value, the dehydration separating effect is not good enough, and production cost is high.Utilizing the methyl alcohol direct dehydrogenation to synthesize anhydrous formaldehyde, then is a kind of new process of remarkable in economical benefits.At first, this method does not have water to generate, and can save rectifying device investment and operating cost thereof; But next by-product high-quality hydrogen, and hydrogen is easy to separate from reaction system.It is this that to produce in the anhydrous formaldehyde method crucial technology by the methyl alcohol direct dehydrogenation partly be the catalyst that is adopted, existing patent report is by the molten zinc of zinc, potassium, indium or the aluminium of the catalyst of being made up of copper, silver, silicon (special public clear 41-11853), fusion or the alloy of these metals (special public clear 47-19251), carbon containing or contain the alloy (spy opens clear 48-97808) of zinc and the catalyst (spy opens clear 52-215) be made up of copper, zinc, selenium etc., but these methods have various shortcoming such as catalyst life is short, reactivity is low, do not have industrial application value.In addition, the catalyst that use is made up of copper, zinc, sulphur (spy opens clear 51-1407) and form catalyst by copper, zinc, supply gas state sulfide carries out the method (spy opens clear 51-76209) of methanol dehydrogenation, sneak into sulphur in reaction product or discharge gas, this do not have industrial prospect to the disagreeableness catalyst of environment.A kind of use of catalyst containing sodium makes the formaldehyde yield bring up to 70% (Appl.Catal.2001 (213) 203), but owing to problems such as reaction temperature and catalyst life and regeneration have limited its industrial applications.
Can test with the following method activity of such catalysts provided by the invention:
The present invention adopts the fixed bed flow method to investigate catalyst activity.By Bubbling method methanol steam (vapourizing temperature is 30~60 ℃) is sent into reactor and contact 450~750 ℃ of reaction temperatures, H in reacted methanol content, content of formaldehyde and the tail gas with catalyst 2, CO and CO 2Content measure by gas chromatography respectively.
Reactor is that an internal diameter is 4.5 millimeters a quartz ampoule, and the novel load silver catalyst that makes places the reaction tube constant temperature zone, and catalytic bed temperature is measured with nickel chromium-nickel silicon thermocouple, and the point for measuring temperature of thermocouple places catalytic bed bottom centre place.
The temperature of the outer electric furnace of conditioned reaction device makes the reaction temperature of catalytic bed be stabilized in required numerical value.Among the present invention, reaction condition should be controlled at appropriate value.The reaction bed temperature is between 450~750 ℃.The catalyst bed layer height is between 5~50mm.The reacting gas air speed is controlled at 1 * 10 4~30 * 10 4Hr -1Between (under the standard state).
Embodiment 1: take by weighing the 0.75g silver nitrate and be dissolved in 100mL water, add 5.56g Mg (NO 3) 26H 2O, treat that dissolving is placed in the 250mL three-necked bottle, dropwise add the 33mL ethyl orthosilicate, simultaneously violent electromagnetic agitation is kept 70 ℃ of oil bath temperatures, and adding 50mL gelatinizing agent~isopropyl alcohol, load onto reflux condensing tube, stir colloidal sol curing after three hours, stop to stir, be warming up to 100 ℃, evaporating solvent 12 hours.Be transferred to a crucible,, be transferred to 700 ℃ of roastings of muffle furnace 6 hours then, be crushed to 40~60 orders, can get the Ag-SiO of silver content 5% with infrared lamp baking 24 hours 2-MgO catalyst is designated as the 1# catalyst.
Embodiment 2: take by weighing the 1.5g silver nitrate and be dissolved in 100mL water, add 5.56g Mg (NO 3) 26H 2O, treat that dissolving is placed in the 250mL three-necked bottle, disposable adding 33mL ethyl orthosilicate, simultaneously violent electromagnetic agitation is kept 75 ℃ of oil bath temperatures, and adding 50mL gelatinizing agent~ethanol, load onto reflux condensing tube, stir colloidal sol curing after six hours, stop to stir, be warming up to 90 ℃, evaporating solvent 24 hours.Be transferred to a crucible,, be transferred to 650 ℃ of roastings of muffle furnace 10 hours then, be crushed to 40~60 orders, can get the Ag-SiO of silver content 10% with infrared lamp baking 24 hours 2-MgO catalyst is designated as the 2# catalyst.


