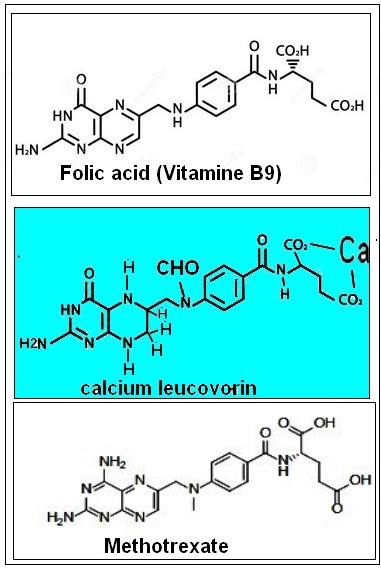
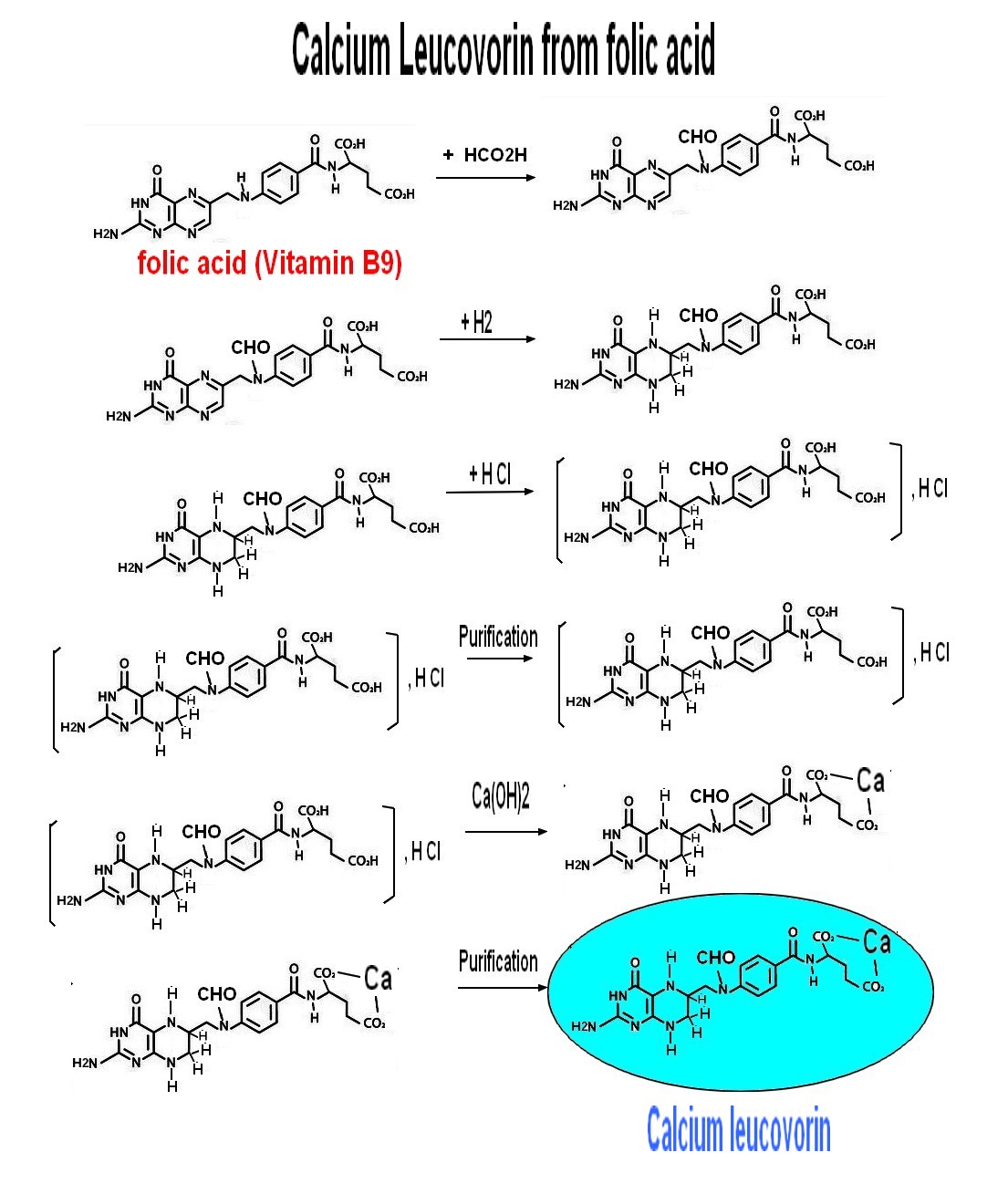
Main use
Leucovorin calcium is a medication used to decrease the toxic effects of methotrexate (methotrexate was first developed in the 1950s as a cancer treatment and is still used for this in higher doses.) . Leucovoein is also used in combination with 5 fluorouracil to treat colorectal cancer and pancreatic cancer, may be used to treat folate deficiency that results in anemia and methanol poisoning .
Commercially available leucovorin is composed of a 1:1 racemic mixture of the dextrorotary and levorotary isomers, while levoleucovorin contains only the pharmacologically active levo-isomer. In vitro, the levo-isomer has been shown to be rapidly converted to the biologically available methyl-tetrahydrofolate form while the dextro form is slowly excreted by the kidneys. Despite this difference in activity, the two commercially available forms have been shown to be pharmacokinetically identical and may be used interchangeably with limited differences in efficacy or side effects .