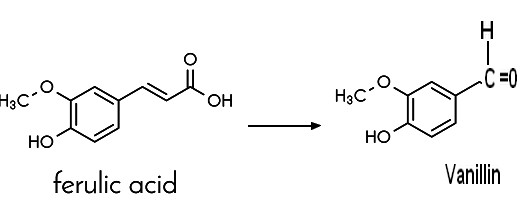

| IL124800A 1998-06-08 Application filed by Givaudan Roure Int Example 1 250 mL shake flasks containing 50 mL of the following medium were prepared: 103 gL"1 sucrose, 4 gL"1 Na2HP04, 1 gL'1 KH2PO4, 1 gL-1 yeast extract, 0.2 gL'1 NaCl, 0.2 gL"1 MgS04 and 0.05 gL"1 CaCl2. The pH was adjusted to 7.2 using NaOH. A shake flask was inoculated with 2 mL of preculture of Streptomyces setonii ATCC 39116 and cultivated at 37°C, 190 rpm for 16 hours. At the end of the growth phase 0.3 g ferulic acid (purchased from Aldrich, cat. no. 12.870-8, 99%) was added to the culture. For this - 8 -purpose a 10 % w/w solution of the acid substrate in 0.5 M NaOH (final pH of the solution was approximately 7.2) was previously prepared and sterile-filtered. The flask was incubated again at 37°C, 190 rpm. After 31.5 hours of biotransformation (incubation) a vanillin concentration of 3.10 L"l (HPLC) was reached. A molecular yield of 66 mol % was calculated. | DE19532317A1 1995-09-01 Haarmann & Reimer Gmbh Example 3 Production of vanillin in a 10 l fermenter 5 l culture medium (4 g / l glucose, 10 g / l malt extract and 6 g / l yeast extract) were sterilized in a fermenter and after cooling with 100 ml of a Pre-culture of DSM 9992 inoculated according to Example 1. The culture conditions were: 37 ° C, 500 rpm, 5 l air / min. 12.5 hours after 1.634 kg of an approximately 3.7% ferulic acid solution (60.2 g Ferulic acid) added. After 17.5 hours over a period of 10 Hours another 4.377 kg of an approximately 3.7% ferulic acid solution (164.72 g Ferula acid) pumped in. The fermentation was stopped after 32 hours. The concentration on Vanillin was 11.5 g / l and 1 g / l of unreacted ferulic acid was still present. The final volume was 11.29 l. This is an implementation of 77.8% of theory based on converted ferulic acid. |
| WO-2022133274-A1
Biosynthesis of vanillin from isoeugenol Basf
Se, Basf Corporation
2020-12-18
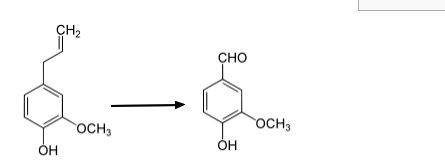
2022-06-23 BACKGROUND OF THE INVENTION Vanilla flavors are among some of the most frequently used flavors worldwide. They are used in the flavorings of numerous foods such as ice cream, dairy products, desserts, confectionary, bakery products and spirits. They are also used in perfumes, pharmaceuticals and personal hygiene products. Natural vanilla flavor has been obtained traditionally from the fermented pods of vanilla orchids. It is formed mainly after the harvest during several weeks of a drying and fermentation process of the beans by hydrolysis of vanillin glucoside that is present in the beans. The essential aromatic substance of vanilla flavor is vanillin (4-hydroxy 3- methoxybenzaldehy de) . Vanillin is one of the most common flavor chemicals and is widely used in the food and beverage, perfume, pharmaceutical, and medical industries. About 12,000 tons of vanillin is consumed annually, of which only 20-50 tons are extracted from vanilla beans, the rest is produced synthetically, mostly from petrochemicals such as guaiacol and lignin. In recent years, increasing demands for natural flavors have led the flavor industry to produce vanillin by bioconversion, as the products of such bioconversion are considered natural by various regulatory and legislative authorities (e.g., European Community Legislation) when produced from biological sources such as living cells or their enzymes, and can be marketed as “natural products”. Natural isoeugenol can be extracted from essential oils and is economical to use for the production of vanillin by enzymatic conversion or microbial bioconversion. Vanillin production via conversion of isoeugenol has been widely reported in a number of microorganisms, including Aspergillus niger, Bacillus subtilis, and Pseudomonas putida. However, the reported titers produced by these microorganisms were very low (less than 2 g/L), significantly limiting the practical application of this approach in the industry. Moreover, the reported bioconversion processes were complicated, further increasing the cost of vanillin production. Accordingly, there is a need in the art for more cost-effective methods for producing vanillin with higher titers and conversion rates. | Example 6: Bioconversion of isoeugenol to vanillin in fermenter
A fermentation process was developed for the bioconversion of
isoeugenol to vanillin using the E. coli strain ISEG-V224 in fermenters.
One ml of glycerol stock of ISEG-V224 was inoculated into 100 mL seed
culture medium (Luria-Bertani medium with 5g/L yeast extract, lOg/L
tryptone, lOg/L NaCl, and 50mg/L kanamycin) in 500 mL flasks. The seed
was cultivated in a shaker with shaking speed of 200rpm at 37 °C for 8
hours and then transferred into 2 liter of fermentation medium of
Luria-Bertani medium plus 6 g/L initial glucose, 50mg/L kanamycin in a 5
-liter fermenter.
The present fermentation process has two phases; namely, a cell growth
phase and a bioconversion phase. The cell growth phase was from 0 hour
to 17 hours and is referred as elapsed fermentation time (EFT). During
EFT, the fermentation parameters were set as follows: Air flow: 0.6vvm;
pH was controlled not to go below 7.1 by using 4N NaOH. The growth
temperature was set to 30°C and the agitation speed was set to 300-500
rpm. The level of dissolved oxygen (DO) was maintained above 30%. At EFT
6.5h, IPTG was added to a final concentration of 0.5mM and glucose was
fed at a rate of 0.4 g/L/hour for 17 hours.
The bioconversion phase was from EFT 17 hour to 46.5 hour. The fermentation parameters were set as follows: Air flow: 0.4vvm. pH was controlled not to go below 8.0 with 4N NaOH, and the temperature was kept at 30°C. Agitation was set to 250-500 rpm and DO was maintained above 30%. The feeding of isoeugenol at a rate of 1.5 g/L began at EFT 17 hour and continued for 4 hours. At EFT 21 hours, the isoeugenol feeding rate was reduced to 1 g/L. At EFT 23 hours, the isoeugenol feeding rate was further reduced to 0.6 g/L/hour and maintained for another 8 hours. Samples were collected at specific time intervals, then analyzed by HPLC as described in Example 4. [00126] Referring to FIG. 6, using a 5-liter fermenter, the E. coli strain ISEG-V224 which was transformed with the UmlEM gene was able to produce vanillin using isoeugenol as the substrate at a titer as high as 14.8 g/L. It is also worth noting that the molar conversion rate from isoeugenol to vanillin reaches above 90%. |
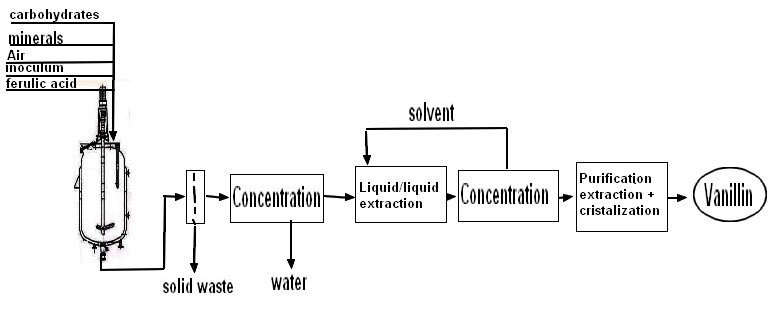
| from US4021493A (1975) It is known that vanillin can be made by oxidizing a lignin or lignosulfonate material, such as results from the kraft and sulfite cooking processes for producing pulp. The oxidation is carried out at elevated temperatures and pressures in the presence of an oxygen-containing gas in an alkaline solution. The resulting alkaline aqueous solution contains in addition to the desired vanillin, the unwanted contaminants, such as orthovanillin, acetovanillone, para-hydroxybenzaldehyde and syringaldehyde, which must be removed from the vanillin if the vanillin is to be of high purity and quality. Numerous processes have been proposed for purifying vanillin from alkaline solutions. These include Sandborn U.S. Pat. No. 2,104,701. This patent treats by countercurrent extraction the aqueous alkaline solution of vanillin with a suitable water-immiscible solvent, such as normal butyl alcohol, and recovers the solvent for reuse. The vanillin is removed from the solvent and is subjected to further purification by any known means, such as by distilling the solvent as its water-binary mixture to leave the vanillin compound in an alkaline aqueous solution for further refining. Other patents employing butyl alcohol extractions include U.S. Pat. Nos. 2,399,607, 2,104,701 and 2,489,200. Other patents employ propyl alcohol or isopropyl alcohol as the extractant, such as U.S. Pat. No. 2,721,221. Other patents disclosed purification of vanillin by employing distillation treatments such as U.S. Pat. Nos. 2,506,540 and 2,745,796. While the foregoing treatments are useful in purifying vanillin, it is desired to provide improved processes which more efficiently and effectively isolate and separate vanillin from its chemically related contaminating impurities. Accordingly, it is an object of the present invention to provide an efficient and effective process for removing and purifying vanillin from aqueous alkaline solutions containing contaminants. | 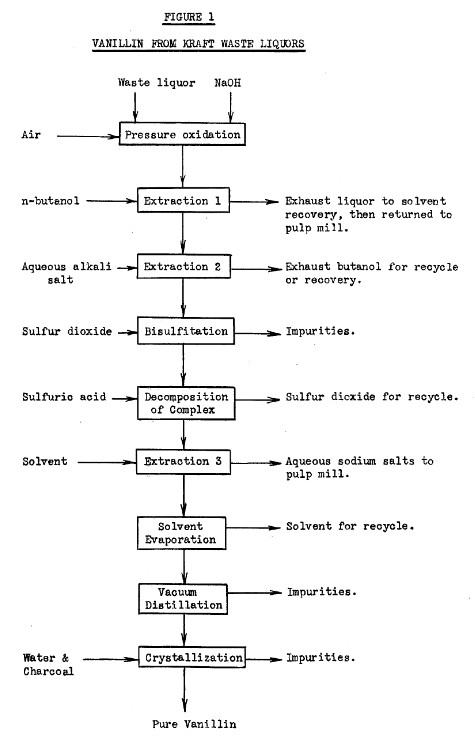 |
| US20240158329A1 (2021) Method for purifying vanillin or derivatives thereof obtained by a biotechnological method Abstract The present invention relates to a process for purifying a fermentation must (M), obtained via a biotechnological process, comprising biomass and vanillin or derivatives thereof in aqueous solution, for the preparation of a crystallized vanillin or derivatives thereof, characterized in that, throughout the purification process, the vanillin or derivatives thereof in protonated or salified form remain in aqueous solution. |
| PROCESS FOR PURIFYING VANILLIN BY LIQUID-LIQUID EXTRACTION FR2984314B1 Abstract translated from French Worldwide applications 2011 FR 2012 A process for the purification of vanillin, from a solution of vanillin in a solvent S1 containing impurities, is described, comprising the following steps: a) a step of evaporation of the solvent S1 in the presence of water to obtain an aqueous solution vanillin; b) a liquid/liquid extraction step by bringing the aqueous solution obtained at the end of step a) into contact with a solvent S2, at a pH greater than 8 and less than 10, to obtain an organic phase and an aqueous phase containing vanillin and residual solvent S2; c) a step of precipitation, at a pH comprised from 4 to 7.5, of the vanillin contained in the aqueous phase obtained at the end of step b), and d) a step of isolation of the vanillin. |
|
CN111548260A * 2020-06-02 2020-08-18
上海欣晨新技术有限公司 Separation method of 6-methyl vanillin and vanillin Separation method of 6-methyl vanillin and vanillin Abstract The invention provides a separation method of vanillin and impurity 6-methyl vanillin, which realizes the good separation of 6-methyl vanillin and vanillin by extracting the mixed solution containing vanillin, 6-methyl vanillin and other impurities with alcohol water; wherein, an appropriate extractant alcohol aqueous solution is selected, and the mass concentration of alcohol in the alcohol aqueous solution is 20-60%; the weight ratio of the solvent for alcohol-water extraction is (0.5-4): 1; furthermore, the method can well realize the effective separation of vanillin, 6-methyl vanillin and other impurities in the vanillin crystallization mother liquor, so that the vanillin finished product obtained in the separation and refining process can meet higher quality requirements, the loss of vanillin in the separation process is reduced, and the yield of vanillin is improved. |
| DE19532317A1
1995-09-01 1997-03-06 Haarmann & Reimer
Gmbh Process for the production of vanillin and suitable
microorganisms Claims (4) translated from German 1. Amycolatopsis sp. from the genus Pseudonocardia with the in the German Collection for Microorganisms and Cell Cultures GmbH in Braunschweig under the numbers DSM 9991 and DSM 9992 Tribes. 2. Process for the preparation of vanillin from ferulic acid in the presence of Amycolatopsis sp. DSM 9991 or DSM 9992 or its enzymes or of microorganisms with genetic material from Amycolatopsis sp. DSM 9991 or DSM 9992, which are the structural and regulatory genes for the Encodes enzymes that are effective in this reaction. 3. The method according to claim 2, according to which natural as the starting component uses ferulic acid. 4. Use of the produced by the method according to claims 2 and 3 Vanillins for the production of flavors. Example 3 Production of vanillin in a 10 l fermenter 5 l culture medium (4 g / l glucose, 10 g / l malt extract and 6 g / l yeast extract) were sterilized in a fermenter and after cooling with 100 ml of a Pre-culture of DSM 9992 inoculated according to Example 1. The culture conditions were: 37 ° C, 500 rpm, 5 l air / min. 12.5 hours after 1.634 kg of an approximately 3.7% ferulic acid solution (60.2 g Ferulic acid) added. After 17.5 hours over a period of 10 Hours another 4.377 kg of an approximately 3.7% ferulic acid solution (164.72 g Ferula acid) pumped in. The fermentation was stopped after 32 hours. The concentration on Vanillin was 11.5 g / l and 1 g / l of unreacted ferulic acid was still present. The final volume was 11.29 l. This is an implementation of 77.8% of theory based on converted ferulic acid. |
| US-10428356-B2 | 2013-11-04 | Methods of making vanillin via the microbial fermentation of ferulic acid from eugenol using a plant dehydrogenase | Bgn Tech Llc |
| US-5573941-A | 1988-03-17 | Callus formation vanilla planifolia | University Of Delaware |
| JP-H0787987-A | 1990-04-19 | Preparation of vanillin by biological conversion of benzenoid precursor | Pernod Ricard Sa, ペルノ・リカルド |
| US-5128253-A | 1991-05-31 | Bioconversion process for the production of vanillin | Kraft General Foods, Inc. |
| JP-H05117125-A | 1991-10-22 | Growth retaining agent for lawn | Dainichiseika Color & Chem Mfg Co Ltd, 大日精化工業株式会社 |
| JP-H05227980-A | 1992-02-21 | Production of vanillin and its related compound by fermentation | Takasago Internatl Corp, 高砂香料工業株式会社 |
| WO-9413614-A1 | 1992-12-10 | Production of vanillin | Quest International B.V. |
| JP-H0759534-A | 1993-08-26 | Production of sweetening seasoning | Kikkoman Corp, キッコーマン株式会社 |
| JP-H07115957-A | 1993-10-21 | Method for improving quality of grain distilling malt liquor | Kikkoman Corp, キッコーマン株式会社 |
| US-5866380-A | 1994-09-13 | Methods for bioconversion of ferulic acid to vanillic acid or vanillin and for the bioconversion of vanillic acid to vanillin using filamentous fungi | Institut National De La Recherche Agronomique-I.N.R.A. |
| JP-H0889230-A | 1994-09-26 | Production of sake | Kikkoman Corp, キッコーマン株式会社 |
| WO-9634971-A1 | 1995-05-05 | Method for producing vanillin using the bioconversion of benzene precursors | Orsan |
| JP-H09206068-A | 1995-09-01 | Production of vanillin and suitable microorganism therefor | Haarmann & Reimer Gmbh, ハーマン・ウント・ライマー・ゲゼルシヤフト・ミツト・ベシユレンクテル・ハフツング |
| JP-H09238675-A | 1996-03-07 | Yeast for new brewing | Sanwa Shiyurui Kk, 三和酒類株式会社 |
| EP-0904396-B1 | 1996-03-23 | Production of vanillin | Plant Bioscience Limited |
| TW-429242-B | 1996-07-22 | Vanillin, ferulic derivatives compounds, and it's vasodilatory vanilloid type β1-adrenoceptior antagonist | Chen Ying Jiun, Lin Dung He |
| DE-19649655-A1 | 1996-11-29 | Synthetic enzymes for the production of coniferyl alcohol, coniferyl aldehyde, ferulic acid, vanillin and vanillic acid and their use | Haarmann & Reimer Gmbh |
| KR-100493538-B1 | 1997-02-07 | Ferulic acid decarboxylase | 교와 핫꼬 고교 가부시끼가이샤 |
| IL-124800-A | 1997-06-19 | Microbiological process for the production of vanillin and guaiacol from ferulic acid | Givaudan Roure Int |
| MX-PA98004891-A | 1997-06-19 | Process for the production of vainill | Givaudanroure (International) Sa |
| JP-2000125840-A | 1998-10-29 | Production of shochu | Kikkoman Corp, キッコーマン株式会社 |
| HU-P0104772-A2 | 1998-10-31 | Construction of production strains for producing substituted phenols by specifically inactivating genes of the eugenol and ferulic acid catabolism | Haarmann & Reimer Gmbh. |
| MX-PA01004338-A | 1998-10-31 | Construction of production strains for producing substituted phenols by specifically inactivating genes of the eugenol and ferulic acid catabolism | Symrise Gmbh&Ampco Kg |
| GB-2347424-A | 1999-02-24 | Peparation of Vanillin Derivatives | Zylepsis Ltd |
| WO-0144480-A2 | 1999-12-14 | Enzymes and genes used for producing vanillin | Haarmann & Reimer Gmbh |
| DE-10144308-A1 | 2001-09-10 | Genetic modification of Amycolatopsis spp., used in producing vanillin, e.g. from ferulic acid, involves contacting culture with transforming and different DNA, magnesium and cesium chloride and polyethylene glycol | Haarmann & Reimer Gmbh |
| US-8053010-B2 | 2001-12-04 | Bran and bran containing products of improved flavor and methods of preparation | General Mills, Inc. |
| CN-1421523-A | 2002-07-22 | Aspergillus niger and its microbial conversion process of producing vanillic acid and vanillic aldehyde | 江南大学, 浙江鑫富生化股份有限公司 |
| US-2006172402-A1 | 2002-10-23 | Production of vanillin in microbial cells | Havkin-Krenkel Daphna J, Gerben Zylstra, Chaim Frenkel, Faith Belanger |
| JP-2009173670-A | 2002-10-30 | Method for producing plant processed product | Suntory Holdings Ltd, サントリーホールディングス株式会社 |
| JP-2004267131-A | 2003-03-10 | Method for producing vanillin by using alkalophilic bacterium | Tsuno Rice Fine Chemicals Co Ltd, 築野ライスファインケミカルズ株式会社 |
| US-2011268858-A1 | 2003-03-28 | Preparation of vanillin from microbial transformation media by extraction by means supercritical fluids or gases | Saf-Isis |
| CN-1544409-A | 2003-08-19 | Ferulic acid and ferulate preparation method | 丽珠集团利民制药厂 |
| CN-1629301-A | 2003-12-18 | Preparation of Vanillin through microorganism conversion | 天津科技大学 |
| JP-2006067812-A | 2004-08-31 | New yeast for brewing | Nanao Junya, 七尾 淳也 |
| US-7462470-B2 | 2005-06-17 | Method for the producing vanillic acid and vanillin from waste residue of rice bran oil by fermentation and biotransformation | Zhejiang Hangzhou Xinfu Pharmaceutical Co., Ltd |
| WO-2007099230-A2 | 2006-03-01 | Expression system for yeast for the production of aromatic molecules | V. Mane Fils |
| CN-101165168-B | 2006-10-20 | Streptomycete and method for producing vanillin by using the same to biologically transform ferulic acid | 上海爱普香料有限公司, 上海凯信生物科技有限公司 |
| FR-2912758-A1 | 2007-02-21 | Production of ferulic acid, coniferyl alcohol and/or natural vanillin, comprises bioconversion of eugenol by a bacterium belonging to genus Streptomyces comprising at least a nucleotide sequence | Mane Fils Sa V |
| WO-2008116319-A1 | 2007-03-27 | Bioproduction of ferulic acid and uses thereof | The Royal Institution For The Advancement Of Learning/Mcgill University |
| ES-2854374-T3 | 2007-04-19 | Procedure to produce vanillin from immobilized microorganisms by surface culture | Laboratorios Minkab S A De C V |
| US-11401535-B2 | 2007-04-19 | Process of production of vanillin with immobilized microorganisms | Laboratorios Minkab, S.A. De C.V. |
| KR-20090076396-A | 2008-01-08 | Vanillin resistant e. coli strain and method for enhancing vanillin production using the strain and adsorbent resin | 경상대학교산학협력단 |
| KR-100918121-B1 | 2008-01-14 | E. coli strain for increasing acetyl-CoA consumption and method of producing vanillin using the strain and adsorbent resin | 경상대학교산학협력단 |
| WO-2010005581-A4 | 2008-07-11 | Niacin compositions for reduction of amyloid beta peptide 42 (abeta 42) production and for treatment of alzheimer's disease (ad) | Kareus Therapeutics, Llc |
| US-2013338199-A1 | 2008-07-11 | Novel Niacin Compositions for Reduction of Amyloid Beta Peptide 42 (AB42) Production and for Treatment of Alzheimer's Disease | Uday Saxena, Venkateswarlu Akella |
| ES-2603745-T3 | 2009-08-21 | Antibacterial food composition | Nutrivercell |
| KR-101163542-B1 | 2010-02-05 | Methods of preparing for Biotransformed Vanillin from Isoeugenol | 광주과학기술원 |
| JP-2011172548-A | 2010-02-26 | Distilled liquor, mirin, and method for producing the same | Kikkoman Corp, キッコーマン株式会社 |
| KR-20120058796-A | 2010-11-30 | Hair cosmetic composition containing natural dyeing material | (주)아모레퍼시픽 |
| ES-2613665-T3 | 2011-02-18 | Procedure to produce a sugar solution | Toray Industries, Inc. |
| CN-102119784-B | 2011-02-23 | Spice extract prepared from waste tobacco leaves and preparation method and application thereof | 红云红河烟草(集团)有限责任公司 |
| CN-102217780-B | 2011-02-23 | Tobacco flavor extract and preparation method and application thereof | 红云红河烟草(集团)有限责任公司 |
| CN-102219727-A | 2011-04-29 | Synthesis method of compound indometacin-feruloyl esterase | 淮阴工学院 |
| EP-2721148-A1 | 2011-06-17 | Microorganisms and methods for producing substituted phenols | Symrise AG |
| CN-102443551-B | 2011-08-19 | Bacillus subtilis and method for producing vanillin with ferulic acid biotransformed by bacillus subtilis | 甘肃省商业科技研究所 |
| US-10351817-B2 | 2011-10-24 | Amycolatopsis sp. strain and methods of using the same for vanillin production | Bgn Tech Llc |
| KR-20130109446-A | 2012-03-27 | Biosensor for detecting phenolic compounds in lignin-hydrolysate and method for detecting phenolic compounds using the same | 국립대학법인 울산과학기술대학교 산학협력단 |
| CN-103215197-A | 2012-04-19 | Enterobacter mori and method for producing natural vanillin by biotransformation of ferulic acid by Enterobacter mori | 湖北中烟工业有限责任公司 |
| CN-102701957-A | 2012-05-21 | Production method of trans-ferulic acid and ester compound | 于志明 |
| CN-102747008-B | 2012-07-23 | Bacillus methylotrophicus VJ4-1 and method for producing natural vanillin by ferulic acid biotransformation with the same | 湖北中烟工业有限责任公司 |
| CN-102851240-A | 2012-09-05 | Raoultella planticola VP4-4 and method for producing natural vanillin through biotransformation of ferulic acid by Raoultella planticola VP4-4 | 湖北中烟工业有限责任公司 |
| KR-20140035187-A | 2012-09-13 | Biosensor for detection of biomass toxic hydrolysate containing furfural compound and detection method using thereof | 국립대학법인 울산과학기술대학교 산학협력단, 한국세라믹기술원 |
| JP-2014057536-A | 2012-09-18 | Raw sake for distilled liquor having high content of 4-vinylguaiacol and method for manufacturing the same; distilled liquor obtained by using the raw sake for distilled liquor; and distilled liquor having high content of vanillin | Suntory Holdings Ltd, サントリーホールディングス株式会社 |
| AU-2017265117-B2 | 2012-11-05 | Vanillin synthase | Danstar Ferment Ag, University Of Copenhagen |
| WO-2014106189-A3 | 2012-12-31 | Methods of making vanillin via microbial fermentation utilizing ferulic acid provided by a modified caffeic acid 3-o-methyltransferase | Conagen Inc., Givaudan Sa |
| CN-103060392-A | 2013-01-22 | Method for converting ferulic acid to produce vanillin by immobilized amycolatopsis | 江南大学 |
| EP-2772142-A1 | 2013-02-27 | Vanillin | Symrise AG |
| CN-103255084-B | 2013-04-23 | Bacillus licheniformis 7172 and application thereof | 南京林业大学 |
| BR-112015029141-B1 | 2013-05-21 | process for extracting ferulic acid, and process for producing natural vanillin | Rhodia Operations |
| CN-103709021-A | 2013-05-29 | Vanillin extraction method | 浙江普洛康裕生物制药有限公司 |
| US-2016145651-A1 | 2013-07-09 | Method of producing sugar liquid | Toray Industries, Inc. |
| MX-2016000929-A | 2013-07-22 | Genetic engineering of pseudomonas putida kt2440 for rapid and high yield production of vanillin from ferulic acid. | Basf Se |
| WO-2015143960-A1 | 2014-03-28 | High xylanase yield aspergillus niger and application thereof | 中国科学院广州能源研究所 |
| CN-104099272-A | 2014-07-09 | Phenolic acid type allelochemical degrading bacterium and preparation and application of microbial inoculum thereof | 山东农业大学 |
| WO-2016050654-A1 | 2014-09-29 | Process for converting ferulic acid into vanillin | Symrise Ag |
| CN-105622394-A | 2014-10-29 | Microwave method ferulic acid synthesis technology | 赵建英 |
| CN-105622395-A | 2014-11-07 | Synthesis process for ferulic acid | 青岛首泰农业科技有限公司 |
| CN-104480148-A | 2014-12-12 | Process for synthesizing vanillin by adopting bio-enzyme catalytic oxidation method | 波顿(上海)生物技术有限公司 |
| CN-104531781-A | 2014-12-12 | Process for synthesizing vanillin by adopting catalytic oxidation method | 波顿(上海)生物技术有限公司 |
| AR-104337-A1 | 2015-05-13 | THERAPEUTIC COMPOSITIONS, METHODS FOR USE AND USES | Ions Pharmaceuticals S À R L |
| EP-3310907-A1 | 2015-06-16 | Molecular cloning and expression of cdna encoding o-methyltransferase isolated from mangifera indica | Council of Scientific and Industrial Research |
| US-2018177184-A1 | 2015-07-21 | Antimicrobial, insecticidal and acaricidal system | Universidad Politecnica De Valencia |
| US-2018142270-A1 | 2015-07-27 | Streptomyces psammoticus and methods of using the same for vanillin production | Xiamen Oamic Biotechnology Co., Ltd. |
| CN-106701884-A | 2016-11-30 | Method for producing natural vanillin based on bioconversion of ferulic acid by use of compound bacteria | 湖北中烟工业有限责任公司 |
| CN-106754773-B | 2016-12-26 | Isoeugenol monooxygenase operon gene, recombinant vector thereof and recombinant pseudomonas | 波顿(上海)生物技术有限公司 |
| CN-108338275-B | 2018-02-10 | Compound additive for improving lactation performance of sows in brackish water areas and use method thereof | 河南雄峰科技股份有限公司 |
| CN-108384738-B | 2018-04-28 | Pseudomonas aeruginosa, screening method thereof and application thereof in straw lactic acid fermentation | 华中农业大学 |
| CN-108753854-B | 2018-06-13 | Method for co-production fermentation of fumaric acid and vanillin | 南京林业大学 |
| CN-110257312-B | 2019-05-06 | Recombinant gene engineering bacterium and application thereof in producing vanillin by fermentation | 浙江工业大学 |
| CN-113088460-B | 2019-12-23 | Amycolatopsis mutant and application thereof | 波顿(上海)生物技术有限公司 |
| WO-2021129233-A1 | 2019-12-25 | Method for preparing natural ferulic acid by using oryzanol-containing saponin as raw material | 湖南华诚生物资源股份有限公司 |
| CN-111066407-B | 2020-01-17 | Method for degrading phenolic acid substances in continuous cropping soil | 河北工程大学 |
| CN-116096857-A | 2020-03-05 | Biosynthesis of commodity chemicals from oil palm empty fruit cluster lignin | 新加坡国立大学 |
| CN-114075163-A | 2020-08-21 | Method for leaching luteolin in perilla stems in hydrothermal acid-controlled manner | 重庆工商大学 |
| EP-4263483-A1 | 2020-12-16 | Processes for the production of vanillin and related compounds | Univerza V Ljubljani |
| US-2024060097-A1 | 2020-12-18 | Bioconversion of ferulic acid to vanillin | Basf Se |
| KR-20230121065-A | 2020-12-18 | Amicolatopsis strain with inhibited vanillic acid formation for vanillin production | 바스프 에스이 |
| CN-113061084-B | 2020-12-31 | Novel method for preparing ferulic acid | 成都亨达药业有限公司, 南京济群医药科技股份有限公司 |
| CN-113061560-A | 2021-03-11 | Genetically engineered bacterium of amycolatopsis as well as construction method and application thereof | 江南大学 |
| EP-4377285-A2 | 2021-07-27 | Method for extracting ferulic acid and/or its salts comprising a step a) in which a biomass is extruded in the presence of a base | Specialty Operations France |
| CN-113637607-A | 2021-08-16 | Amycolatopsis and application thereof | 西安海斯夫生物科技有限公司 |
| CN-113583912-B | 2021-08-24 | Enterobacter cholerae and application thereof in vanillin production | 四川轻化工大学 |
| CN-118369147-A | 2021-10-01 | Extraction and purification of natural ferulic acid from biomass and conversion to vanillin | 斯佩罗可再生能源有限责任公司 |
| CN-113755422-B | 2021-10-09 | Recombinant amycolatopsis capable of highly producing vanillin, construction method and application thereof | 陕西海斯夫生物工程有限公司 |
| CN-116059265-A | 2021-11-04 | Application of ligusticum chuanxiong hort ethanol extract in preparation of medicine with liver protection effect | 西南大学, 重庆悦本草生物技术开发有限公司 |
| CN-113957017-A9 | 2021-11-29 | Bacillus subtilis and method for producing vanillin by using bacillus subtilis | 内蒙古昆明卷烟有限责任公司 |
| CN-114437963-B | 2021-12-27 | Streptomyces olive and application thereof in biosynthesis of vanillin | 四川盈嘉合生科技有限公司 |
| CN-114058521-B | 2022-01-17 | Trichoderma atroviride C61 | 山东省科学院生态研究所(山东省科学院中日友好生物技术研究中心) |
| CN-115184491-A | 2022-07-05 | Method for measuring chemical component content in Qingjin Yiqi granules | 天津中医药大学 |
| CN-115166112-A | 2022-07-08 | High performance liquid detection method for ferulic acid and vanillin in continuous cropping soil | 安徽科技学院 |
| CN-117902961-A | 2022-10-12 | Method for preparing vanillin by taking ferulic acid as raw material | 桂林莱茵生物科技股份有限公司 |
| CN-115927489-A | 2022-10-27 | Method for synthesizing aromatic compound by biological catalysis | 上海交通大学 |
| CN-115927152-A | 2022-12-09 | Synthesis and accumulation of vanillin in genetic engineering bacterium escherichia coli | 深圳中科欣扬生物科技有限公司 |
| CN-115948315-A | 2022-12-09 | Construction of high-yield vanillin engineering bacterium escherichia coli | 深圳中科欣扬生物科技有限公司 |
| CN-117305194-B | 2023-03-10 | Amycolatopsis mutant strain and application thereof | 江南大学 |
| WO-2024197188-A2 | 2023-03-21 | Production and purification of natural vanillin using new strains of amycolatopsis | Spero Renewables, Llc |
| CN-116676208-B | 2023-03-22 | Bacillus coagulans and application thereof | 南京林业大学 |
| CN-116349675-A | 2023-04-04 | Spartina alterniflora inhibitor and application thereof | 上海大学 |
| CN-116640712-A | 2023-04-19 | Method for constructing vanillin self-regulating bi-directional transport system in escherichia coli to improve vanillin yield | 中国农业科学院农产品加工研究所 |
| CN-116590161-B | 2023-05-10 | Recombinant Amycolatopsis producing vanillin, construction method and application thereof | 陕西海斯夫生物工程有限公司 |
| CN-116925941-A | 2023-06-30 | Pichia pastoris engineering bacteria using ferulic acid as substrate and application thereof in vanillin production | 中国农业科学院农产品加工研究所 |
| CN-117247858-B | 2023-07-06 | Microbial agent for reducing continuous cropping obstacle of tobacco and improving quality and increasing yield as well as preparation method and application thereof | 云南农业大学 |
| CN-117025647-A | 2023-07-21 | Method for synthesizing ethyl ferulate | 北京理工大学 |
| KR-102669025-B1 | 2023-10-23 | Lactiplantibacillus plantarum strain with excellent phenolic acid decarboxylase activity and use thereof | 샘표식품 주식회사 |
| CN-117603896-A | 2023-11-23 | Genetically engineered bacterium for synthesizing tyrosine and derivatives thereof, construction method and application thereof | 四川盈嘉合生科技有限公司 |
| CN-117741032-A | 2023-12-20 | Separation method of chemical components in corn silk and preparation thereof or construction method of characteristic spectrum | 华润三九现代中药制药有限公司 |
| CN-117777254-A | 2023-12-28 | Expression module, recombinant strain and application thereof | 四川盈嘉合生科技有限公司 |
| CN-117904216-A | 2024-01-23 | Method for improving vanillin yield | 天津大学合成生物前沿研究院 |
| CN-118360262-A | 2024-05-08 | Carotenoid cleavage oxygenase mutant and application thereof | 陕西师范大学 |
| CN-118374463-B | 2024-05-23 | 4-Vinyl guaiacol oxygenase mutant for synthesizing vanillin and engineering bacteria thereof | 上海智峪生物科技有限公司 |
| CN-118620854-A | 2024-05-23 | Iso mutant, genetic engineering strain and application thereof in vanillin synthesis | 上海智峪生物科技有限公司 |
| CN-119193649-A | 2024-10-22 | A construction process of a high-vanillin-producing pseudoctenobiotic | 昆山亚香香料股份有限公司 |
| DE-32914-C | Process for the preparation of m-methoxy-p-nitrobenzaldehyde and ferulic acid for the production of vanillin | M. ULRICH in Genf, Rue de Mole 1 |